Orphenadrine Citrate (orphenadrine citrate 100 mg) Dailymed
Generic: orphenadrine citrate is used for the treatment of Urinary Bladder Neck Obstruction Esophageal Achalasia Glaucoma Infant Intestinal Obstruction Muscle Cramp Muscle Rigidity Myasthenia Gravis Myositis Pain Peptic Ulcer Prostatic Hyperplasia Spasm Tetanus
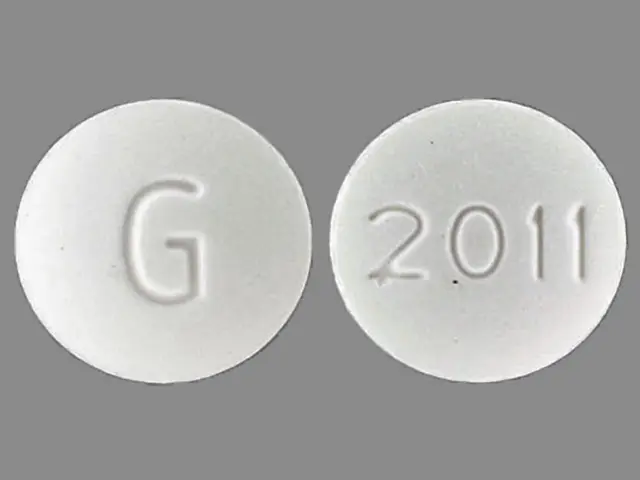
IMPRINT: G 2011
SHAPE: round
COLOR: white
All Imprints
12 hr orphenadrine citrate 100 mg extended release oral tablet - g 2011 round white
orphenadrine citrate 100 mg - g 2011 round white
Go PRO for all pill images
Rx only
Description
Orphenadrine citrate is the citrate salt of orphenadrine (2-dimethylaminoethyl-2-methylbenzhydryl ether citrate). It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol.
Each orphenadrine citrate extended-release tablet contains 100 mg orphenadrine citrate, USP. Orphenadrine citrate extended-release tablets also contain ethylcellulose NF, povidone USP, lactose monohydrate NF, and magnesium stearate NF.
Clinicalpharmacology
The mode of therapeutic action has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense skeletal muscles in man.
Orphenadrine citrate also possesses anti-cholinergic actions.
Indications And Usage
Orphenadrine citrate extended-release tablets are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculo skeletal conditions.
Contraindications
Contraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardio-spasm (megaesophagus) and myasthenia gravis.
Contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
Warnings
Some patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
Precautions
Confusion, anxiety and tremors have been reported in few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Orphenadrine citrate should be used with caution in patients with tachycardia, cardiac decompensation, coronary insufficiency, cardiac arrhythmias.
Safety of continuous long-term therapy with orphenadrine has not been established. Therefore, if orphenadrine is prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with orphenadrine citrate. It is also not know whether orphenadrine citrate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Orphenadrine citrate should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Adverse Reactions
Adverse reactions of orphenadrine are mainly due to the mild anti-cholinergic action of orphenadrine, and are usually associated with higher dosage. Dryness of the mouth is usually the first adverse effect to appear. When the daily dose is increased, possible adverse effects include: tachycardia, palpitation, urinary hesitancy or retention, blurred vision, dilation of pupils, increased ocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, hypersensitivity reactions, pruritus, hallucinations, agitation, tremor, gastric irritation, and rarely urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of mental confusion. These adverse reactions can usually be eliminated by reduction in dosage. Very rare cases of aplastic anemia associated with the use of orphenadrine tablets have been reported. No causal relationship has been established.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Abuse Anddependence
Orphenadrine has been chronically abused for its euphoric effects.[1] The mood elevating effects may occur at therapeutic doses of orphenadrine.[2]
Overdosage
Orphenadrine is toxic when overdosed and typically induces anticholinergic effects.[3] In a review of orphenadrine toxicity, the minimum lethal dose was found to be 2 grams to 3 grams for adults; however, the range of toxicity is variable and unpredictable.[4] Treatment for orphenadrine overdose is evacuation of stomach contents (when necessary), charcoal at repeated doses, intensive monitoring, and appropriate supportive treatment of any emergent anticholinergic effects.[5]
Dosage And Administration
Orphenadrine citrate extended-release tablets: Adults - Two tablets per day; one in the morning and one in the evening.
How Supplied
Orphenadrine Citrate Extended-release Tablets, 100 mg - Each round, white, convex tablet imprinted with “G” on one side and “2011” on the other side.
They are available as follows:
Bottles of 100:Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â NDC 0115-2011-01
Bottles of 500:Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â NDC 0115-2011-02
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Dispense in tightly-closed, light-resistant container (USP).
Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807
124-05
Rev. 01-2019-00
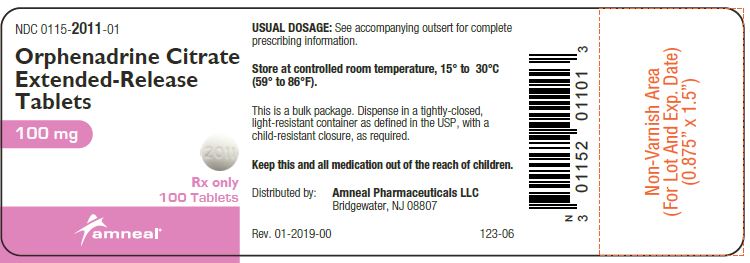
Principal Display Panel - 100 Mg Tablet Bottle Label
NDC 0115-2011-01
Orphenadrine Citrate Extended-Release Tablets
100 mg
Rx only
100 TABLETS
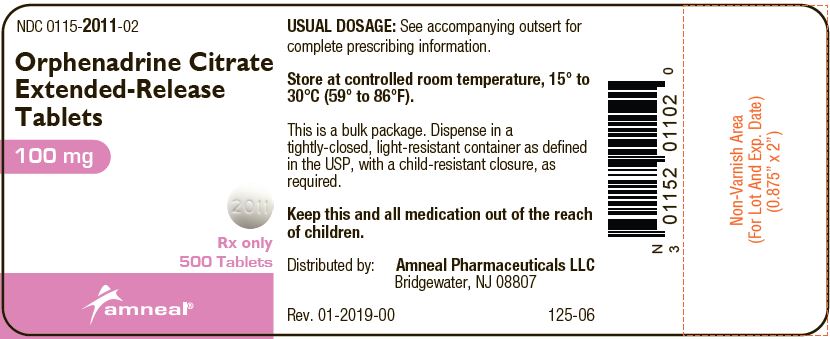
Principal Display Panel - 100 Mg Tablet Bottle Label
NDC 0115-2011-02
Orphenadrine Citrate Extended-Release Tablets
100 mg
Rx only
500 TABLETS
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site