BiDil (hydralazine hydrochloride 37.5 mg isosorbide dinitrate 20 mg) Dailymed
Generic: hydralazine hydrochloride and isosorbide dinitrate is used for the treatment of Coronary Artery Disease Heart Failure Hypersensitivity Hypertension, Malignant Hypertension, Pulmonary Rheumatic Heart Disease Anemia Angina Pectoris Cerebral Hemorrhage Coronary Disease Cyanosis Gastroesophageal Reflux Craniocerebral Trauma Poisoning Esophageal Spasm, Diffuse Glaucoma, Angle-Closure
IMPRINT: 20 N
SHAPE: round
COLOR: orange SCORE: 2
All Imprints
hydralazine hydrochloride 37.5 mg / isosorbide dinitrate 20 mg oral tablet [bidil] - n 20 round orange
hydralazine hydrochloride 37.5 mgisosorbide dinitrate 20 mg - 20 n round orange
Go PRO for all pill images
1 Indications And Usage
BiDil is a combination of isosorbide dinitrate, a nitrate vasodilator, and hydralazine hydrochloride, an arteriolar vasodilator, indicated for:
- the treatment of heart failure as an adjunct therapy to standard therapy in self-identified black patients to improve survival, prolong time to hospitalization for heart failure and to improve patient-reported functional status (
1.1 )
Limitations of use:
- There is little experience in patients with NYHA class IV heart failure (
1.2 )1.1 Treatment of Heart Failure in Self-identified Black Patients
BiDil is indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival, to prolong time to hospitalization for heart failure, and to improve patient-reported functional status.
1.2 Limitations of Use
There is little experience in patients with NYHA class IV heart failure.
2 Dosage And Administration
BiDil should be initiated at a dose of one BiDil Tablet, three times a day. Titrate to a maximum of two tablets three times daily, if tolerated.
Although titration of BiDil can be rapid (3-5 days), some patients may experience side effects and may take longer to reach their maximum tolerated dose. The dosage may be decreased to as little as one-half BiDil Tablet three times a day if intolerable side effects occur. Efforts should be made to titrate up as soon as side effects subside.
One tablet three times a day titrated to a maximum tolerated dose up to two tablets three times a day (2 )
Dosage may be decreased to as little as one-half tablet three times a day if intolerable side effects occur. Efforts should be made to titrate up as soon as side effects subside (2 )
3 Dosage Forms And Strengths
The BiDil (20 mg isosorbide dinitrate and 37.5 mg hydralazine hydrochloride) tablets are orange, biconvex, approximately 8 mm in diameter, scored, film-coated, and debossed with "20" on one side over the score and "N" on the other side.
Tablets (scored): 20 mg isosorbide dinitrate and 37.5 mg hydralazine hydrochloride (3 )
4 Contraindications
BiDil is contraindicated in patients who are allergic to organic nitrates.
Do not use BiDil in patients who are taking PDE-5 inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia [see Drug Interactions (7.1)].
Do not use BiDil in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension.
- Patients who are allergic to organic nitrates (
4 )- Use of phosphodiesterase type 5 (PDE5) inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil, or soluble guanylate cyclase (sGC) stimulator (riociguat). (
4 )
5 Warnings And Precautions
- May cause symptomatic hypotension (
5.1 )- Symptomatic Lupus Erythematosus Syndromes: Consider discontinuation if clinically appropriate (
5.2 )- Myocardial ischemia and angina (
5.3 )- Peripheral Neuritis: May be treated with Pyridoxine (
5.4 )5.1Hypotension
Symptomatic hypotension, particularly with upright posture, may occur with even small doses of BiDil. Hypotension is most likely to occur in patients who have been volume or salt depleted; correct prior to initiation of BiDil [see Adverse Reactions (6.1)].
5.2Systemic Lupus Erythematosus
Hydralazine hydrochloride has been reported to cause a drug-induced systemic lupus erythematosus (SLE) syndrome. Symptoms and signs usually regress when hydralazine hydrochloride is discontinued.
5.3Worsening Ischemic Heart Disease
Hydralazine hydrochloride can cause tachycardia and hypotension potentially leading to myocardial ischemia and angina, particularly in patients with hypertrophic cardiomyopathy.
5.4Peripheral Neuritis
Hydralazine hydrochloride has been associated with peripheral neuritis, evidenced by paresthesia, numbness, and tingling, which may be related to an antipyridoxine effect. Pyridoxine should be added to BiDil therapy if such symptoms develop.
6 Adverse Reactions
Most common adverse reactions (≥ 5% more on BiDil than on placebo) were headache and dizziness (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Arbor Pharmaceuticals, LLC at 1-866-516-4950 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
BiDil has been evaluated for safety in 517 heart failure patients in A-HeFT. A total of 317 of these patients received BiDil for at least 6 months, and 220 received BiDil for at least 12 months. In A-HeFT, 21% of the patients discontinued BiDil for adverse reactions compared to 12% who discontinued placebo. Overall, adverse reactions were more common in BiDil -treated than in placebo-treated patients. Table 1 uls adverse reactions reported with an incidence, after rounding, ≥ 2% higher on BiDil than on placebo in A-HeFT, regardless of causality. The most common reasons for discontinuing BiDil in the A-HeFT trial was headache (7%).
Table 1. Adverse Reactions Occurring in the A-HeFT Study in ≥ 2% of Patients Treated with BiDil. BiDil(N=517)% Placebo(N=527)% Headache 50 21 Dizziness 32 14 Asthenia 14 11 Nausea 10 6 Hypotension 8 4 Sinusitis 4 2 Ventricular tachycardia 4 2 Paresthesia 4 2 Vomiting 4 2 Amblyopia 3 1
In the V-HeFT I and II clinical studies, a total of 587 patients with heart failure were treated with the combination of isosorbide dinitrate and hydralazine hydrochloride. The type, pattern, frequency and severity of adverse reactions reported in these studies were similar to those reported in A-HeFT, described above and no unusual adverse reactions were reported.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of BiDil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Use of BiDil: The following adverse reactions have been identified with use of BiDil.
 Cardiac Disorders: Palpitations Ear and labyrinth disorders: Tinnitus, vertigo Eye Disorders: Eyelid edema, vision blurred Gastrointestinal Disorders: Abdominal discomfort, constipation General Disorders and Administration Site Conditions: Facial pain, flushing, chest discomfort, chest pain, peripheral edema Musculoskeletal and Connective Tissue Disorders: Pain in extremity, myalgia Nervous Disorders: Dysgeusia, hypoaesthesia, migraine, syncope Renal and Urinary Disorders: Chromaturia, pulmonary renal syndrome Respiratory, Thoracic and Mediastinal Disorders: Dyspnea Reproductive System and Breast Disorders: Erectile dysfunction Skin and Subcutaneous Tissue Disorders: Erythema, hyperhidrosis, pruritus, face swelling
Use of Hydralazine Hydrochloride or Isosorbide Dinitrate: The following reactions have been reported with use of either hydralazine hydrochloride or isosorbide dinitrate.
 Blood and Lymphatic System Disorders: Blood dyscrasias, agranulocytosis, purpura, eosinophilia, splenomegaly. Eye Disorders: Lacrimation, conjunctivitis. Gastrointestinal Disorders: Paralytic ileus. Hepatobiliary Disorders: Hepatitis. Psychiatric Disorders: Psychotic reactions, disorientation. Renal and Urinary Disorders: Difficulty in urination.
7 Drug Interactions
7.1Phosphodiesterase Inhibitors
BiDil is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), PDE5 inhibitors such as avanafil, sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use BiDil in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension [see Contraindications (4)].
8 Use In Specific Populations
- Geriatric Use: Isosorbide dinitrate and hydralazine may be eliminated more slowly in elderly patients. Initiate therapy at the low end of the dosing range (
8.5 )8.1 Pregnancy
Risk Summary
There are no data on BiDil use in pregnant women, and insufficient data on its components (hydralazine and isosorbide dinitrate) to assess a drug-associated risk of major birth defects or miscarriage with first trimester use. Available published data on hydralazine use in pregnancy during the second and third trimesters have not shown an association with adverse pregnancy-related outcomes.
Hydralazine hydrochloride is teratogenic in mice at 66 mg/kg and possibly in rabbits at 33 mg/kg (2 and 3 times the MRHD of BiDil on a body surface area basis).
Isosorbide dinitrate has been shown to cause a dose-related increase in embryo-toxicity (excess mummified pups) in rabbits at 70 mg/kg (12 times the MRHD of BiDil on a body surface area basis).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women with heart failure are at increased risk for preterm birth. Clinical classification of heart disease may worsen with pregnancy and lead to maternal death and/or stillbirth.
8.2 Lactation
Risk Summary
There are no data on the presence of BIDIL in human or animal milk, the effects on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BIDIL and any potential adverse effects on the breastfed child from BIDIL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of BiDil in children have not been established.
8.5 Geriatric Use
Clinical studies of BiDil did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic and renal function, and of concomitant disease or other drug therapies.
Isosorbide dinitrate, its active metabolites, and hydralazine may be eliminated more slowly in elderly patients.
8.6 Renal Impairment
There are no studies of renal impairment using BiDil. No dose adjustment is required for hydralazine or isosorbide dinitrite [see Clinical Pharmacology (12.3)].
Dialyzability of hydralazine has not been determined. Dialysis is not an effective method for removing isosorbide dinitrate or its metabolite isosorbide-5-mononitrate from the body.
8.7 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of hydralazine alone has not been determined. Isosorbide dinitrate concentrations increase in patients with cirrhosis. There are no studies of hepatic impairment using BiDil.
10 Overdosage
The signs and symptoms of overdosage with BiDil are expected to be those of excessive pharmacologic effect, i.e., vasodilatation, reduced cardiac output and hypotension, and signs and symptoms include headache, confusion, tachycardia, and generalized skin flushing. Complications can include myocardial ischemia and subsequent myocardial infarction, cardiac arrhythmia, and profound shock. Syncope, coma and death may ensue without appropriate treatment.
Human Experience: There are no documented cases of overdosage with BiDil. No deaths from acute poisoning have been reported.
Treatment: There is no specific antidote. Support of the cardiovascular system is of primary importance. Shock should be treated with plasma expanders, vasopressors, and positive inotropic agents. The gastric contents should be evacuated, taking adequate precautions to prevent aspiration. These manipulations have to be carried out after cardiovascular status has been stabilized, since they might precipitate cardiac arrhythmias or increase the depth of shock.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of isosorbide dinitrate overdose in these patients may be difficult, and invasive monitoring may be required.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of the components of BiDil. Dialysis is not effective in removing circulating isosorbide dinitrate. The dialyzability of hydralazine has not been determined.
Methemoglobinemia: Nitrate ions liberated during metabolism of isosorbide dinitrate can oxidize hemoglobin into methemoglobin. There are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. Methemoglobin levels are measurable by most clinical laboratories. Methemoglobinemia could be serious in chronic heart failure patients because of already compromised vascular bed-tissue gas exchange dynamics. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air. When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1 to 2 mg/kg intravenously.
11 Description
BiDil is a fixed-dose combination of isosorbide dinitrate, a vasodilator with effects on both arteries and veins, and hydralazine hydrochloride, a predominantly arterial vasodilator.
Isosorbide dinitrate is described chemically as 1,4:3,6-dianhydro-D-glucitol dinitrate and its structural formula is:
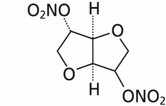
Isosorbide dinitrate is a white to off-white, crystalline powder with the empirical formula C6H8N2O8 and a molecular weight of 236.14. It is freely soluble in organic solvents such as alcohol, chloroform and ether, but is only sparingly soluble in water.
Hydralazine hydrochloride is described chemically as 1-hydrazinophthalazine monohydrochloride, and its structural formula is:
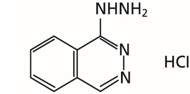
Hydralazine hydrochloride is a white to off-white, crystalline powder with the empirical formula C8H8N4∙HCl and a molecular weight of 196.64. It is soluble in water, slightly soluble in alcohol, and very slightly soluble in ether.
Each BiDil Tablet for oral administration contains 20 mg of isosorbide dinitrate and 37.5 mg of hydralazine hydrochloride.
The inactive ingredients in BiDil tablets include: anhydrous lactose, microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate, hypromellose, FD&C Yellow No. 6 aluminum lake, polyethylene glycol, titanium dioxide, polysorbate 80.
12 Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action underlying the beneficial effects of BiDil in the treatment of heart failure has not been established.
Isosorbide dinitrate is a vasodilator affecting both arteries and veins. Its dilator properties result from the release of nitric oxide and the subsequent activation of guanylyl cyclase, and ultimate relaxation of vascular smooth muscle.
Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of chronically-delivered nitrates. In the large majority of these trials, active agents were no more effective than placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours is response to nitrates restored.
Hydralazine hydrochloride is a selective dilator of arterial smooth muscle. Animal data suggests that hydralazine may also mitigate tolerance to nitrates.
12.2 Pharmacodynamics
The basis for the beneficial clinical effects of BiDil is not known. In a small study of patients with chronic heart failure administered single doses of hydralazine 75 mg, isosorbide dinitrate 20 mg, and the combination, the combination elicited a statistically significant decrease in pulmonary capillary wedge pressure compared to hydralazine alone. The increase in cardiac output, renal blood flow and limb blood flow with the combination, however, was not greater than with hydralazine alone. There is no study of hemodynamic effects following multiple dosing.
12.3 Pharmacokinetics
Absorption
BiDil: Following a single 75-mg oral dose of hydralazine plus 40 mg of isosorbide dinitrate to 19 healthy adults, peak plasma concentrations of hydralazine (88 ng/mL/65 kg) and isosorbide dinitrate (76 ng/mL/65 kg) were reached in 1 hour. The half-lives were about 4 hours for hydralazine and about 2 hours for isosorbide dinitrate. Peak plasma concentrations of the two active metabolites, isosorbide-2-mononitrate and isosorbide-5-mononitrate, were 98 and 364 ng/mL/65 kg, respectively, at about 2 hours. No information is currently available regarding the effect of food on the bioavailability of hydralazine or isosorbide dinitrate from BiDil tablets.
Hydralazine hydrochloride: About 2/3 of a 50-mg dose of 14C-hydralazine hydrochloride given in gelatin capsules was absorbed in hypertensive subjects. In patients with heart failure, mean absolute bioavailability of a single oral dose of hydralazine 75 mg varies from 10 to 26%, with the higher percentages in slow acetylators. Administration of doses escalating from 75 mg to 1000 mg three times daily to congestive heart failure patients resulted in an up to 9-fold increase in the dose normalized AUC, indicating non-linear kinetics of hydralazine, probably reflecting saturable first pass metabolism.
Isosorbide dinitrate: Absorption of isosorbide dinitrate from tablets after oral dosing is nearly complete. The average bioavailability of isosorbide dinitrate is about 25%, but is highly variable (10%-90%) because of first-pass metabolism, and increases progressively during chronic therapy. Serum concentrations reach their maximum about one hour after ingestion.
Distribution
Hydralazine hydrochloride: After intravenous administration of hydralazine in a dose of 0.3 mg/kg, the steady-state volume of distribution in patients with congestive heart failure was 2.2 L/kg.
Isosorbide dinitrate: The volume of distribution of isosorbide dinitrate is 2 to 4 L/kg. About 28% of circulating isosorbide dinitrate is protein bound.
Under steady-state conditions, isosorbide dinitrate accumulates significantly in muscle (pectoral) and vein (saphenous) wall relative to simultaneous plasma concentrations.
Metabolism
Hydralazine is metabolized by acetylation, ring oxidation and conjugation with endogenous compounds including pyruvic acid. Acetylation occurs predominantly during the first-pass after oral administration which explains the dependence of the absolute bioavailability on the acetylator phenotype. About 50% of patients are fast acetylators and have lower exposure.
After oral administration of hydralazine, the major circulating metabolites are hydralazine pyruvate hydrazone and methyltriazolophthalazine. Hydralazine is the main pharmacologically active entity; hydralazine pyruvate hydrazone has only minimal hypotensive and tachycardic activity. The pharmacological activity of methyltriazolophthalazine has not been determined. The major identified metabolite of hydralazine excreted in urine is acetylhydrazinophthalazinone.
Isosorbide dinitrate undergoes extensive first-pass metabolism in the liver and is cleared at a rate of 2 to 4 L/minute with a serum half-life of about 1 hour. Isosorbide dinitrate's clearance is primarily by denitration to the 2-mononitrate (15 to 25%) and the 5-mononitrate (75 to 85%). Both metabolites have biological activity, especially the 5-mononitrate which has an overall half-life of about 5 hours. The 5-mononitrate is cleared by denitration to isosorbide, glucuronidation to the 5-mononitrate glucuronides, and by denitration/hydration to sorbitol. The 2-mononitrate appears to participate in the same metabolic pathways with a half-life of about 2 hours.
Elimination
Hydralazine: Metabolism is the main route for the elimination of hydralazine. Negligible amounts of unchanged hydralazine are excreted in urine.
Isosorbide dinitrate: Most isosorbide dinitrate is eliminated renally as conjugated metabolites.
Specific Populations
No pharmacokinetic studies in special populations were conducted with BiDil. Pharmacokinetics in special populations is based on individual components.
Geriatric Patients -The pharmacokinetics of hydralazine and isosorbide dinitrate, alone or in combination, have not been determined in patients over 65 years of age.
Pediatric Patients - The pharmacokinetics of hydralazine and isosorbide dinitrate, alone or in combination, have not been determined in patients below the age of 18 years.
Gender - There are no studies of gender-dependent effects with hydralazine. In a single dose study with isosorbide dinitrate, no gender-dependent differences in the pharmacokinetics of isosorbide dinitrate and its mononitrate metabolites were found.
Renal Impairment - The effect of renal impairment on the pharmacokinetics of hydralazine has not been determined. In a study with 49 hypertensive patients on chronic therapy with hydralazine in daily doses of 25-200 mg, the daily dose of hydralazine in 19 subjects with severely impaired renal function (creatinine clearance 5-28 mL/min) and in 17 subjects with normal renal function (creatinine clearance >100 mL/min) using a population PK approach was not different, suggesting no need for dose adjustment in patients with renal impairment. The dialyzability of hydralazine has not been determined. In three studies, renal insufficiency did not affect the pharmacokinetics of isosorbide dinitrate. Dialysis is not an effective method for removing isosorbide dinitrate or its metabolite isosorbide-5-mononitrate from the body.
Hepatic Impairment - The effect of hepatic impairment on the pharmacokinetics of hydralazine alone has not been determined. Isosorbide dinitrate concentrations increase in patients with cirrhosis.
Drug-Drug Interactions
No pharmacokinetic drug-drug interaction studies were conducted with BiDil.
Hydralazine: Administration of hydralazine can increase the exposure to a number of drugs including beta-blockers. In healthy males administered a single oral dose of hydralazine 50 mg and propranolol 1 mg/kg, the Cmax and AUC for propranolol approximately doubled. In healthy subjects administered a single oral dose of hydralazine 50 mg and metoprolol 100 mg, the Cmax and AUC for metoprolol increased by 50% and 30%, respectively. In pre-eclamptic women, twice-daily doses of hydralazine 25 mg and metoprolol 50 mg increased the Cmax and AUC for metoprolol by 90% and 40%, respectively.
In healthy males administered single oral doses of hydralazine 25 mg and either lisinopril 20 mg or enalapril 20 mg, Cmax and AUC for lisinopril were each increased 30%, but enalapril concentrations were unaffected.
Intravenous co-administration of 0.2 mg/kg hydralazine HCl and 40 mg furosemide in Japanese patients with congestive heart failure resulted in a 20% increase in the clearance of furosemide.
Isosorbide dinitrate: The vasodilating effects of coadministered isosorbide dinitrate may be additive to those of other vasodilators, including alcohol.
A single dose of 20 mg of isosorbide dinitrate was administered to healthy subjects after pretreatment with 80 mg propranolol three times daily for 48 hours, resulting in no impact on the pharmacokinetics of isosorbide dinitrate and isosorbide-5-mononitrate.
When single 100-mg oral doses of atenolol were administered 2 hours before isosorbide dinitrate at a 10-mg dose no differences in the pharmacokinetics of isosorbide dinitrate or its mononitrates were observed.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Hydralazine hydrochloride: An increased incidence of lung tumors (adenomas and adenocarcinomas) was observed in a lifetime study in Swiss albino mice given hydralazine hydrochloride continuously in their drinking water at a dosage of about 250 mg/kg per day (6 times the MRHD provided by BiDil on a body surface area basis). In a 2-year carcinogenicity study of rats given hydralazine hydrochloride by gavage at dose levels of 15, 30, and 60 mg/kg/day (up to 3 times the MRHD of BiDil on a body surface area basis), microscopic examination of the liver revealed a small, but statistically significant increase in benign neoplastic nodules in males (high-dosage) and females (both high and intermediate dosage groups). Benign interstitial cell tumors of the testes were also significantly increased in the high-dose group.
Hydralazine hydrochloride is mutagenic in bacterial systems, and is positive in rat and rabbit hepatocyte DNA repair studies in vitro. Additional in vivo and in vitro studies using lymphoma cells, germinal cells, fibroblasts from mice, bone marrow cells from Chinese hamsters and fibroblasts from human cell lines did not demonstrate any mutagenic or clastogenic potential for hydralazine hydrochloride.
Isosorbide dinitrate: No long-term animal studies have been performed to evaluate the mutagenic or carcinogenic potential of isosorbide dinitrate. A modified two-litter reproduction study among rats fed isosorbide dinitrate at 25 or 100 mg/kg/day (up to 9 times the Maximum Recommended Human Dose of BiDil on a body surface area basis) revealed no evidence of altered fertility or gestation.
14 Clinical Studies
BiDil or a combination of isosorbide dinitrate and hydralazine hydrochloride was studied in two placebo-controlled clinical trials in 1,692 patients with mild to severe heart failure (mostly NYHA class II and III) and one active control trial (vs. enalapril) in 804 patients. The results of the trials follow:
Placebo-controlled Study : In the multicenter trial V-HeFT I, the combination of hydralazine and isosorbide dinitrate 75 mg/40 mg 4 times daily (n=186) was compared to placebo (n=273) in men with impaired cardiac function and reduced exercise tolerance (primarily NYHA class II and III) and on therapy with digitalis glycosides and diuretics. There was no overall significant difference in mortality between the two treatment groups. There was, however, a trend favoring hydralazine and isosorbide dinitrate, which on retrospective analysis, was attributable to an effect in blacks (n=128). Survival in white patients (n=324) was similar on placebo and the combination treatment.
Active-controlled Study: In a second study of mortality, V-HeFT II, the combination of hydralazine and isosorbide dinitrate 75 mg/40 mg 4 times daily was compared to enalapril in 804 men with impaired cardiac function and reduced exercise tolerance (NYHA class II and III), and on therapy with digitalis glycosides and diuretics. The combination of hydralazine and isosorbide dinitrate was inferior to enalapril overall, but retrospective analysis showed that the difference was observed in the white population (n=574); there was essentially no difference in the black population (n=215).
Based on these retrospective analyses suggesting an effect on survival in black patients, but showing little evidence of an effect in the white population, a third study was conducted among black patients with heart failure.
Placebo-controlled Study: The A-HeFT trial evaluated BiDil vs. placebo among 1,050 self-identified black patients (over 95% NYHA class III) at 169 centers in the United States. All patients had stable symptomatic heart failure. Patients were required to have LVEF ≤ 35% or left ventricular internal diastolic dimension > 2.9 cm/m2 plus LVEF < 45%. Patients were maintained on stable background therapy and randomized to BiDil (n=518) or placebo (n=532). BiDil was initiated at 20 mg isosorbide dinitrate/37.5 mg hydralazine hydrochloride three times daily and titrated to a target dose of 40/75 mg three times daily or to the maximum tolerated dose. Patients were treated for up to 18 months.
The randomized population was 60% male, 1% NYHA class II, 95% NYHA class III and 4% NYHA class IV, with a mean age of 57 years, and was generally treated with standard treatments for heart failure including diuretics (94%, almost all loop diuretics), beta-blockers (87%), angiotensin converting enzyme inhibitors (ACE-I; 78%), angiotensin II receptor blockers (ARBs; 28%), either ACE-I or ARB (93%), digitalis glycosides (62%) and aldosterone antagonists (39%).
The primary endpoint was a composite score consisting of all-cause mortality, first hospitalization for heart failure, and responses to the Minnesota Living with Heart Failure questionnaire. The trial was terminated early, at a mean follow-up of 12 months, primarily because of a statistically significant 43% reduction in all-cause mortality in the BiDil-treated group (p=0.012; see Table 2 and Figure 1). The primary endpoint was also statistically in favor of BiDil (p ≤ 0.021). The BiDil-treated group also showed a 39% reduction in the risk of a first hospitalization for heart failure (p<0.001; see Table 2 and Figure 2) and had statistically significant improvement in response to the Minnesota Living with Heart Failure questionnaire, a self-report of the patient's functional status, at most time points (see Figure 3). Patients in both treatment groups had mean baseline questionnaire scores of 51 (out of a possible 105).
Table 2. Results of A-HeFT (Intent-To-Treat Population) BiDil(N=518) Placebo(N=532) Hazard Ratio(95% CI) P Composite -0.16±1.93 -0.47±2.04 — 0.021 All-cause mortality 6.2% 10.2% 0.57(0.37, 0.89) 0.012 Hospitalization for heart failure 16.4% 24.4% 0.61(0.46, 0.80) <0.001 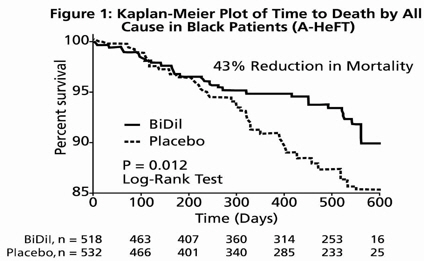
Effects on survival and hospitalization for heart failure were similar in subgroups by age, gender, baseline disease, and use of concomitant medications, as shown in Figure 4.
Patients treated with BiDil in the A-HeFT study had randomly measured blood pressures on average 3/3 mmHg lower than did patients on placebo. The contribution of the difference in blood pressure to the overall outcome difference is unknown. Whether both hydralazine and isosorbide dinitrate contribute to the overall outcome difference has not been studied in outcome trials. Isosorbide dinitrate and hydralazine have not been systematically studied for the treatment of heart failure as separate agents, and neither drug is indicated for heart failure.
16 How Supplied/storage And Handling
BiDil Tablets contain 20 mg of isosorbide dinitrate and 37.5 mg of hydralazine hydrochloride. They are biconvex, approximately 8 mm in diameter, scored, film-coated, orange tablets debossed "20" on one side over the score and "N" on the other side.
- NDC 24338-010-09: Bottles of 90
STORAGE AND HANDLING SECTION
Store at 25°C (77°F), excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature.] Keep bottles tightly closed.
Protect from light. Dispense in a light-resistant, tight container.
17 Patient Counseling Information
Patients should be informed of possible side effects and advised to take the medication regularly and continuously as directed.
Headache
Inform patients that headaches often accompany treatment with BiDil, especially during initiation of treatment. Advise patients to consult a physician to adjust the dose of BiDil if headache continues with repeated dosing.
Hypotension
Warn patients about lightheadedness on standing.
Advise patients that inadequate fluid intake or excessive fluid loss from perspiration, diarrhea or vomiting may lead to an excessive fall in blood pressure and cause lightheadedness or even syncope. If syncope does occur, advise patients to discontinue BiDil and notify their prescribing physician as soon as possible.
Phosphodiesterase-5 Inhibitors
Advise patients to inform their physicians if they are taking, or planning to take, sildenafil, vardenafil, or tadalafil. Bidil should not be taken concomitantly with phosphodiesterase-5 inhibitors.
Worsening Ischemic Heart Disease
Advise patients to inform their physicians of any worsening of symptoms of myocardial ischemia, especially those with hypertrophic cardiomyopathy.
Systemic Lupus Erythematosus-like Symptoms
Advise patients if symptoms suggestive of systemic lupus erythematosus—such as arthralgia, fever, chest pain, prolonged malaise—occur to notify their prescribing physician.
Peripheral Neuritis
Advise patients if symptoms of peripheral neuritis—paresthesia, numbness, and tingling—occur to notify the prescribing physician.
Manufactured for:arbor® PHARMACEUTICALS, LLCAtlanta, GA 30328
Manufactured by:Lannett Company, Inc.Philadelphia, PA 19136
BiDil is a registered trademark of Arbor Pharmaceuticals, LLC
© 2021U.S. Patent number 6,784,177; 6,465,463All rights reserved
BDL-PI-06
Principal Display Panel - 90 Tablet Bottle Label
NDC 24338-010-0990 tablets
BiDil® isosorbide dinitrate and hydralazine HCl tablets
20 mg/37.5 mg
Store at room temperature 15°C - 30°C (59°F - 86°F)
BDL-TL-09-00 Rev. 09/20CIB71996B
Rx only

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


