Clindamycin (clindamycin hydrochloride 150 mg) Dailymed
Generic: clindamycin is used for the treatment of Acne Vulgaris Actinomycetales Infections Bacteroides Infections Endocarditis, Bacterial Enterocolitis, Pseudomembranous Pneumonia, Pneumocystis Staphylococcal Infections Streptococcal Infections Toxoplasmosis Vaginosis, Bacterial Liver Failure
Go PRO for all pill images
For Use In Dogs Only
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Clindamycin Hydrochloride Capsules, USP contain clindamycin hydrochloride which is the hydrated salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chlorosubstitution of the 7(R)-hydroxyl group of a naturally produced antibiotic produced by Streptomyces lincolnensis var. lincolnensis. Clindamycin Hydrochloride Capsules, USP (For Use in Dogs Only):
25 mg Capsule, each yellow and colorless capsule contains clindamycin hydrochloride equivalent to 25 mg of clindamycin.
75 mg Capsule, each green and colorless capsule contains clindamycin hydrochloride equivalent to 75 mg of clindamycin.
150 mg Capsule, each blue and colorless capsule contains clindamycin hydrochloride equivalent to 150 mg of clindamycin.
300 mg Capsule, each turquoise and colorless capsule contains clindamycin hydrochloride equivalent to 300 mg of clindamycin.
Clinical Pharmacology
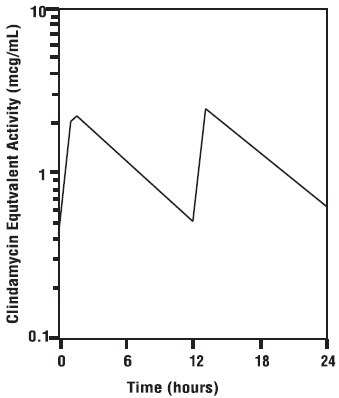
Absorption Clindamycin hydrochloride is rapidly absorbed from the canine gastrointestinal tract. Dog Serum Levels Serum levels at or above 0.5 µg/mL can be maintained by oral dosing at a rate of 2.5 mg/lb of clindamycin hydrochloride every 12 hours. This same study revealed that average peak serum concentrations of clindamycin occur 1 hour and 15 minutes after oral dosing. The elimination half -life for clindamycin in dog serum was approximately 5 hours. There was no bioactivity accumulation after a regimen of multiple oral doses in healthy dogs. Clindamycin Serum Concentrations
2.5 mg/lb (5.5 mg/kg)ÂAfter B.I.D. Oral
Dose of Clindamycin Hydrochloride Capsules to Dogs
Metabolism and Excretion
Extensive studies of the metabolism and excretion of clindamycin hydrochloride administered orally in animals and humans have shown that unchanged drug and bioactive and bioinactive metabolites are excreted in urine and feces. Almost all of the bioactivity detected in serum after clindamycin hydrochloride product administration is due to the parent molecule (clindamycin). Urine bioactivity, however, reflects a mixture of clindamycin and active metabolites, especially N-demethyl clindamycin and clindamycin sulfoxide. Site and Mode of Action Clindamycin is an inhibitor of protein synthesis in the bacterial cell. The site of binding appears to be in the 50S sub-unit of the ribosome. Binding occurs to the soluble RNA fraction of certain ribosomes, thereby inhibiting the binding of amino acids to those ribosomes. Clindamycin differs from cell wall inhibitors in that it causes irreversible modification of the protein-synthesizing subcellular elements at the ribosomal level. Microbiology Clindamycin is a lincosaminide antimicrobial agent with activity against a wide variety of aerobic and anaerobic bacterial pathogens. Clindamycin is a bacteriostatic compound that inhibits bacterial protein synthesis by binding to the 50S ribosomal sub-unit. The minimum inhibitory concentrations (MICs) of Gram-positive and obligate anaerobic pathogens isolated from dogs in the United States are presented in Table 1. Bacteria were isolated in 1998-1999. All MICs were performed in accordance with the Clinical and Laboratory Standards Institute (CLSI).
Table 1. Clindamycin MIC Values (µg/mL) from Diagnostic Laboratory Survey Data Evaluating Canine Pathogens in the U.S. during 1998-991 Organism Number of Isolates MIC50 MIC85 MIC90 Range Soft Tissue/Wound2 Staphylococcus aureus 17 0.5 0.5 ≥ 4.0 0.25- ≥ 4.0 Staphylococcus intermedius 28 0.25 0.5 ≥ 4.0 0.125- ≥ 4.0 Staphylococcus spp 18 0.5 0.5 ≥ 4.0 0.25- ≥ 4.0 Beta-hemolytic streptococci 46 0.5 0.5 ≥ 4.0 0.25- ≥ 4.0 Streptococcus spp 11 0.5 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Osteomyelitis/Bone3 Staphylococcus aureus 20 0.5 0.5 0.5 0.54 Staphylococcus intermedius 15 0.5 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Staphylococcus spp 18 0.5 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Beta-hemolytic streptococci 21 0.5 2.0 2.0 0.25-≥ 4.0 Streptococcus spp 21 ≥ 4.0 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Dermal/Skin5 Staphylococcus aureus 25 0.5 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Staphylococcus intermedius 48 0.5 ≥ 4.0 ≥ 4.0 0.125- ≥ 4.0 Staphylococcus spp 32 0.5 ≥ 4.0 ≥ 4.0 0.25-≥ 4.0 Beta-hemolytic streptococci 17 0.5 0.5 0.5 0.25-0.5
1 The correlation between the in vitro susceptibility data and clinical response has not been determined.
2 Soft Tissue/Wound: includes samples labeled wound, abscess, aspirate, exudates, draining tract, lesion, and mass.
3 Osteomyelitis/Bone: includes samples labeled bone, fracture, joint, tendon4 No range, all isolates yielded the same value5 Dermal/Skin: includes labeled skin, skin swab, biopsy, incision, lip
Indications
Clindamycin Hydrochloride Capsules, USP (for use in dogs only) are indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the specific conditions uled below: Dogs: Skin infections (wounds and abscesses) due to coagulase positive staphylococci (Staphylococcus aureus or Staphylococcus intermedius). Deep wounds and abscesses due to Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens. Dental infections due to Staphylococcus aureus, Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens. Osteomyelitis due to Staphylococcus aureus, Bacteroides fragilis, Prevotella melaninogenicus, Fusobacterium necrophorum and Clostridium perfringens.
Contraindications
Clindamycin Hydrochloride Capsules, USP are contraindicated in animals with a history of hypersensitivity to preparations containing clindamycin or lincomycin. Because of potential adverse gastrointestinal effects, do not administer to rabbits, hamsters, guinea pigs, horses, chinchillas or ruminating animals.
Warnings
Keep out of reach of children. Not for human use.
Precautions
During prolonged therapy of one month or greater, periodic liver and kidney function tests and blood counts should be performed. The use of Clindamycin Hydrochloride Capsules, USP occasionally results in overgrowth of non-susceptible organisms such as clostridia and yeasts. Therefore, the administration of Clindamycin Hydrochloride Capsules should be avoided in those species sensitive to the gastrointestinal effects of clindamycin (see CONTRAINDICATIONS). Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation. Patients with very severe renal disease and/or very severe hepatic disease accompanied by severe metabolic aberrations should be dosed with caution, and serum clindamycin levels monitored during high-dose therapy. Clindamycin hydrochloride has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, Clindamycin Hydrochloride Capsules, USP should be used with caution in animals receiving such agents. Safety in gestating bitches and queens or breeding male dogs has not been established.
Adverse Reactions
Side effects occasionally observed in either clinical trials or during clinical use were vomiting and diarrhea. To report a suspected adverse reaction call 1-844-227-6687 (1-844-2-CRONUS)
Dosage And Administration
Dogs: Infected Wounds, Abscesses, and Dental Infections Oral: 2.5-15.0 mg/lb body weight every 12 hours. Duration: Treatment with Clindamycin Hydrochloride Capsules, USP may be continued up to a maximum of 28 days if clinical judgment indicates. Treatment of acute infections should not be continued for more than three or four days if no response to therapy is seen. Dosage Schedule
Capsules Clindamycin Hydrochloride Capsules, USPÂ 25 mg, administer 1-6 capsules every 12 hours for each 10 pounds of body weight. Clindamycin Hydrochloride Capsules, USP 75 mg, administer 1 -6 capsules every 12 hours for each 30 pounds of body weight. Clindamycin Hydrochloride Capsules, USPÂ 150 mg, administer 1-6 capsules every 12 hours for each 60 pounds of body weight. Clindamycin Hydrochloride Capsules, USPÂ 300 mg, administer 1-6 capsules every 12 hours for each 120 pounds of body weight. Dogs: Osteomyelitis Oral: 5.0-15.0 mg/lb body weight every 12 hours. Duration: Treatment with Clindamycin Hydrochloride Capsules is recommended for a minimum of 28 days. Treatment should not be continued for longer than 28 days if no response to therapy is seen. Dosage Schedule:
Capsules Clindamycin Hydrochloride Capsules, USP 25 mg, administer 2-6 capsules every 12 hours for each 10 pounds of body weight. Clindamycin Hydrochloride Capsules, USP 75 mg, administer 2 -6 capsules every 12 hours for each 30 pounds of body weight. Clindamycin Hydrochloride Capsules, USP 150 mg, administer 2-6 capsules every 12 hours for each 60 pounds of body weight. Clindamycin Hydrochloride Capsules, USP 300 mg, administer 2-6 capsules every 12 hours for each 120 pounds of body weight
Animal Safety Summary
Rat and Dog Data: One year oral toxicity studies in rats and dogs at doses of 30, 100 and 300 mg/kg/day (13.6, 45.5 and 136.4 mg/lb/day) have shown clindamycin hydrochloride capsules to be well tolerated. Differences did not occur in the parameters evaluated to assess toxicity when comparing groups of treated animals with contemporary controls. Rats administered clindamycin hydrochloride at 600 mg/kg/day (272.7 mg/lb/day) for six months tolerated the drug well; however, dogs orally dosed at 600 mg/kg/day (272.7 mg/lb/day) vomited, had anorexia, and subsequently lost weight. At necropsy these dogs had erosive gastritis and focal areas of necrosis of the mucosa of the gallbladder. Safety in gestating bitches or breeding males has not been established.
Storage
Store at controlled room temperature 68° to 77°F (20° to 25°C).
How Supplied Clindamycin Hydrochloride Capsules are available as: 25 mg -  bottles of 200 75 mg - bottles of 200 150 mg - bottles of 100 and 500 300 mg - bottles of 100 To report suspected adverse drug events, for technical assistance or to obtain a copy of the safety data sheet (SDS), contact Cronus Pharma LLC at 1-844-227-6687 or 1-844-2-CRONUS.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at https://www.fda.gov/animal-veterinary/safety-health.
Approved by FDA under ANADA # 200-298 Manufactured for: Cronus Pharma LLC,East Brunswick, NJ 08816.Contact No: 1-844-227-6687(1-844-2-CRONUS) Made in India Distributed by: Cronus Pharma LLC 2 Tower Center Boulevard, Suite 1101A, East Brunswick, NJ 08816 Contact No: 1-844-227-6687 (1-844-2-CRONUS) e-Fax No: 732-647-1272 Email: contact@cronuspharma.com Issued: 02/2023
Package Label.principal Display Panel
NDC 69043-013-20
Clindamycin Hydrochloride Capsules, USP
25 mg
Equivalent to 25 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
200 Capsules
For Use in Dogs Only

Package Label.principal Display Panel
NDC 69043-014-20
Clindamycin Hydrochloride Capsules, USP
75 mg
Equivalent to 75 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
200 Capsules
For Use in Dogs Only
Package Label.principal Display Panel
NDC 69043-015-01
Clindamycin Hydrochloride Capsules, USP
150 mg
Equivalent to 150 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
100 Capsules
For Use in Dogs Only

Package Label.principal Display Panel
NDC 69043-015-05
Clindamycin Hydrochloride Capsules, USP
150 mg
Equivalent to 150 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
500 Capsules
For Use in Dogs Only

Package Label.principal Display Panel
NDC 69043-016-01
Clindamycin Hydrochloride Capsules, USP
300 mg
Equivalent to 300 mg clindamycin
Approved by FDA under ANADA # 200-298
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
100 Capsules
For Use in Dogs Only
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


