CitraNatal Essence (ascorbic acid 120 mg iron 35 mg cholecalciferol 400 [iu] alpha-tocopherol 30 [iu] riboflavin 3.4 mg niacinamide 20 mg pyridoxine hydrochloride 25 mg folic acid 1 mg iodine 150 ug zinc 25 mg copper 2 mg thiamine 3 mg calcium citrate 140 mg) Dailymed
Boxed Warning
Warning
Boxed Warning
Warning
Boxed Warning
Warning
Go PRO for all pill images
Description
CitraNatal Essence™ is a prescription prenatal/postnatal multi-vitamin/ mineral tablet with Ferr-Ease®, a dual-iron delivery comprising both a quick release and slow release iron, and a soft gel of DHA, an essential fatty acid. The prenatal vitamin is a white, coated, oval multi-vitamin/mineral tablet. The tablet is debossed “0864” on one side and is blank on the other. The essential fatty acid DHA soft gel is oblong, transparent, and caramel colored.
Indications
CitraNatal Essence TM is a multivitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.
Contraindications
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Warning
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
Warning
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
Warning
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12 is deficient.
Precautions
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Adverse Reactions
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Dosage And Administration
One tablet and one capsule daily or as directed by a physician.
Storage
Store at 20–25°C (68–77°F)
NOTICE
Contact with moisture can discolor or erode the tablet.
To report a serious adverse event or obtain product information, call (210) 696-8400.
51065R0420
Mission ® PHARMACAL
DHA capsules manufactured for: MISSION PHARMACAL COMPANY San Antonio, TX USA 78230 1355 Prenatal tablets manufactured by: MISSION PHARMACAL COMPANY San Antonio, TX USA 78230 1355
Copyright © 2020 Mission Pharmacal Company.
All rights reserved.
www.missionpharmacal.com
Ferr-Ease ® Dual-iron delivery
Trademark of Mission Pharmacal Company U.S. Patent No. 6,521,247
life's DHA ®
life’sDHA is a trademark of DSM. U.S. Patent No. 7,579,174 U.S. Patent No. 7,732,170 U.S. Patent No. 5,518,918
*March of Dimes does not endorse specific products or brands. March of Dimes is a registered trademark of the March of Dimes Foundation.
How Supplied Section
Six child-resistant buler packs of 5 tablets and 5 capsules each - NDC 0178-0864-30
Package Label.principal Display Panel
Retail Carton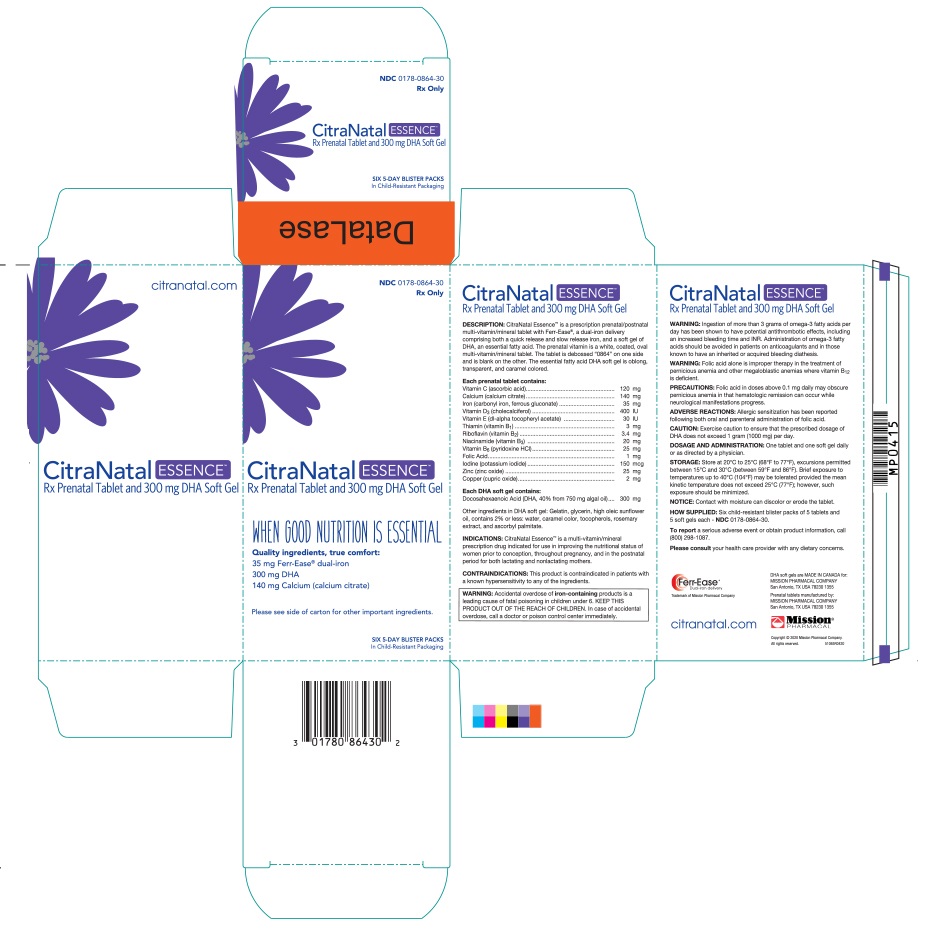
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

