CLONAZEPAM (clonazepam 0.5 mg) Dailymed
Generic: clonazepam is used for the treatment of Bipolar Disorder Dysarthria Epilepsies, Myoclonic Epilepsy, Absence Liver Diseases Neuralgia Pregnancy Restless Legs Syndrome Schizophrenia Seizures Tic Disorders Glaucoma, Angle-Closure Panic Disorder Lennox Gastaut Syndrome
Boxed Warning
Warning: Risks From Concomitant Use With Opioids; Abuse, Misuse, And Addiction; And Dependence And Withdrawal Reactions
Go PRO for all pill images

Rx only
Warning: Risks From Concomitant Use With Opioids; Abuse, Misuse, And Addiction; And Dependence And Withdrawal Reactions
‚Äʬ†Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death.¬† ‚Äʬ†Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. ‚Äʬ†Limit dosages and durations to the minimum required. ‚Äʬ†Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS). ‚Äʬ†The use of benzodiazepines, including clonazepam orally disintegrating tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing clonazepam orally disintegrating tablets and throughout treatment, assess each patient‚Äôs risk for abuse, misuse, and addiction (see WARNINGS). ‚Äʬ†The continued use of benzodiazepines, including clonazepam orally disintegrating tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of clonazepam orally disintegrating tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam orally disintegrating tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
Description
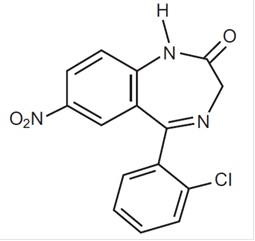
Clonazepam, USP a benzodiazepine, is available as an orally disintegrating tablet containing 0.125 mg, 0.25 mg, 0.5 mg, 1 mg or 2 mg clonazepam, USP. Each orally disintegrating tablet also contains sorbitol, aspartame, sodium lauryl sulfate, crospovidone, mannitol, colloidal silicon dioxide, talc and magnesium stearate. Chemically, clonazepam, USP is 5-(2-chlorophenyl)-1,3-dihydro-7-nitro-2H-1,4-­benzodiazepin-2-one. It is a light yellow powder. It has a molecular weight of 315.72 and the following structural formula:
Clinical Pharmacology
Pharmacodynamics: The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown, although it is believed to be related to its ability to enhance the activity of gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Convulsions produced in rodents by pentylenetetrazol or, to a lesser extent, electrical stimulation are antagonized, as are convulsions produced by photic stimulation in susceptible baboons. A taming effect in aggressive primates, muscle weakness and hypnosis are also produced. In humans, clonazepam is capable of suppressing the spike and wave discharge in absence seizures (petit mal) and decreasing the frequency, amplitude, duration and spread of discharge in minor motor seizures.
Pharmacokinetics: Clonazepam is rapidly and completely absorbed after oral administration. The absolute bioavailability of clonazepam is about 90%. Maximum plasma concentrations of clonazepam are reached within 1 to 4 hours after oral administration. Clonazepam is approximately 85% bound to plasma proteins. Clonazepam is highly metabolized, with less than 2% unchanged clonazepam being excreted in the urine. Biotransformation occurs mainly by reduction of the 7-nitro group to the 4-amino derivative. This derivative can be acetylated, hydroxylated and glucuronidated. Cytochrome P-450 including CYP3A, may play an important role in clonazepam reduction and oxidation. The elimination half-life of clonazepam is typically 30 to 40 hours. Clonazepam pharmacokinetics are dose-independent throughout the dosing range. There is no evidence that clonazepam induces its own metabolism or that of other drugs in humans.
Pharmacokinetics in Demographic Subpopulations and in Disease States:  Controlled studies examining the influence of gender and age on clonazepam pharmacokinetics have not been conducted, nor have the effects of renal or liver disease on clonazepam pharmacokinetics been studied. Because clonazepam undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Thus, caution should be exercised when administering clonazepam to these patients.
Clinical Trials: Panic Disorder : The effectiveness of clonazepam orally disintegrating tablets in the treatment of panic disorder was demonstrated in two double-blind, placebo-controlled studies of adult outpatients who had a primary diagnosis of panic disorder (DSM-IIIR) with or without agoraphobia. In these studies, clonazepam orally disintegrating tablets was shown to be significantly more effective than placebo in treating panic disorder on change from baseline in panic attack frequency, the Clinician’s Global Impression Severity of Illness Score and the Clinician’s Global Impression Improvement Score.
Study 1 was a 9-week, fixed-dose study involving clonazepam orally disintegrating tablets doses of 0.5, 1, 2, 3 or 4 mg/day or placebo. This study was conducted in four phases: a 1-week placebo lead-in, a 3-week upward titration, a 6-week fixed dose and a 7-week discontinuance phase. A significant difference from placebo was observed consistently only for the 1 mg/day group. The difference between the 1 mg dose group and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 74% of patients receiving clonazepam 1 mg/day were free of full panic attacks, compared to 56% of placebo-treated patients.
Study 2 was a 6-week, flexible-dose study involving clonazepam orally disintegrating tablets in a dose range of 0.5 to 4 mg/day or placebo. This study was conducted in three phases: a 1-week placebo lead-in, a 6-week optimal-dose and a 6-week discontinuance phase. The mean clonazepam dose during the optimal dosing period was 2.3 mg/day. The difference between clonazepam orally disintegrating tablets and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 62% of patients receiving clonazepam were free of full panic attacks, compared to 37% of placebo-treated patients.
Subgroup analyses did not indicate that there were any differences in treatment outcomes as a function of race or gender.
Indications Andusage
Seizure Disorders: Clonazepam orally disintegrating tablet is useful alone or as an adjunct in the treatment of the Lennox-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures. In patients with absence seizures (petit mal) who have failed to respond to succinimides, clonazepam orally disintegrating tablets may be useful.
In some studies, up to 30% of patients have shown a loss of anticonvulsant activity, often within 3 months of administration. In some cases, dosage adjustment may reestablish efficacy.
Panic Disorder: Clonazepam orally disintegrating tablet is indicated for the treatment of panic disorder, with or without agoraphobia, as defined in DSM-V. Panic disorder is characterized by the occurrence of unexpected panic attacks and associated concern about having additional attacks, worry about the implications or consequences of the attacks, and/or a significant change in behavior related to the attacks.
The efficacy of clonazepam orally disintegrating tablets was established in two 6- to 9-week trials in panic disorder patients whose diagnoses corresponded to the DSM-IIIR category of panic disorder (see CLINICAL PHARMACOLOGY: Clinical Trials).
Panic disorder (DSM-V) is characterized by recurrent unexpected panic attacks, ie, a discrete period of intense fear or discomfort in which four (or more) of the following symptoms develop abruptly and reach a peak within 10 minutes: (1) palpitations, pounding heart or accelerated heart rate; (2) sweating; (3) trembling or shaking; (4) sensations of shortness of breath or smothering; (5) feeling of choking; (6) chest pain or discomfort; (7) nausea or abdominal distress; (8) feeling dizzy, unsteady, lightheaded or faint; (9) derealization (feelings of unreality) or depersonalization (being detached from oneself); (10) fear of losing control; (11) fear of dying; (12) paresthesias (numbness or tingling sensations); (13) chills or hot flushes.
The effectiveness of clonazepam orally disintegrating tablets in long-term use, that is, for more than 9 weeks, has not been systematically studied in controlled clinical trials. The physician who elects to use clonazepam orally disintegrating tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
Contraindications
Clonazepam orally disintegrating tablets are contraindicated in patients with the following conditions:
- History of sensitivity to benzodiazepines
- Clinical or biochemical evidence of significant liver disease
- Acute narrow angle glaucoma (it may be used in patients with open angle glaucoma who are receiving appropriate therapy).
Warnings
Risks from Concomitant Use With Opioids: Concomitant use of benzodiazepines, including clonazepam orally disintegrating tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe clonazepam orally disintegrating tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when clonazepam orally disintegrating tablet is used with opioids (see PRECAUTIONS: Information for Patients and PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction:
The use of benzodiazepines, including clonazepam orally disintegrating tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing clonazepam orally disintegrating tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of clonazepam orally disintegrating tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of clonazepam orally disintegrating tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective  dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions:
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam orally disintegrating tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of CLONAZEPAM ORALLY DISINTEGRATING TABLETS).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including clonazepam orally disintegrating tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of clonazepam orally disintegrating tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Interference With Cognitive and Motor Performance: Since clonazepam orally disintegrating tablets produces CNS depression, patients receiving this drug should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery or driving a motor vehicle. They should also be warned about the concomitant use of alcohol or other CNS-depressant drugs during clonazepam orally disintegrating tablets therapy (see PRECAUTIONS: Drug Interactions and Information for Patients).
Suicidal Behavior and Ideation: Antiepileptic drugs (AEDs), including clonazepam orally disintegrating tablets, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono-and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43% compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1: Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
Indication Placebo Patients with Events Per 1000 Patients Drug Patients with Events Per 1000 Patients Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients Risk Difference: Additional Drug Patients with Events per 1000 Patients Epilepsy 1 3.4 3.5 2.4 Psychiatric 5.7 8.5 1.5 2.9 Other 1 1.8 1.9 0.9 Total 2.4 4.3 1.8 1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing clonazepam orally disintegrating tablets or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Neonatal Sedation and Withdrawal Syndrome: Use of clonazepam orally disintegrating tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see PRECAUTIONS: Pregnancy). Monitor neonates exposed to clonazepam orally disintegrating tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to clonazepam orally disintegrating tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.
Precautions
General:
Worsening of Seizures : When used in patients in whom several different types of seizure disorders coexist, clonazepam orally disintegrating tablets may increase the incidence or precipitate the onset of generalized tonic-clonic seizures (grand mal). This may require the addition of appropriate anticonvulsants or an increase in their dosages. The concomitant use of valproic acid and clonazepam orally disintegrating tablets may produce absence status.
Laboratory Testing During Long-Term Therapy:  Periodic blood counts and liver function tests are advisable during long-term therapy with clonazepam orally disintegrating tablets.  
Caution in Renally Impaired Patients:  Metabolites of clonazepam orally disintegrating tablets are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function.
Hypersalivation : Clonazepam orally disintegrating tablets may produce an increase in salivation. This should be considered before giving the drug to patients who have difficulty handling secretions.
Respiratory Compromise:  Clonazepam orally disintegrating tablets should be used with caution in patients with compromised respiratory function.
Porphyria:  Clonazepam orally disintegrating tablets may have a porphyrogenic effect and should be used with care in patients with porphyria.
Information for Patients:
A clonazepam orally disintegrating tablets Medication Guide must be given to the patient each time clonazepam orally disintegrating tablets are dispensed, as required by law. Patients should be instructed to take clonazepam orally disintegrating tablets only as prescribed. Physicians are advised to discuss the following issues with patients for whom they prescribe clonazepam orally disintegrating tablets:
Risks from Concomitant Use With Opioids : Inform patients and caregivers that potentially fatal additive effects may occur if clonazepam orally disintegrating tablet is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider (see WARNINGS: Risks from Concomitant Use With Opioids and PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction: Inform patients that the use of clonazepam orally disintegrating tablets, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS: Abuse, Misuse, and Addiction and DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions: Inform patients that the continued use of clonazepam orally disintegrating tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of clonazepam orally disintegrating tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of clonazepam orally disintegrating tablets may require a slow taper (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE).
Interference With Cognitive and Motor Performance:Because benzodiazepines have the potential to impair judgment, thinking or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that clonazepam orally disintegrating tablets therapy does not affect them adversely.
Suicidal Thinking and Behavior:Patients, their caregivers, and families should be counseled that AEDs, including clonazepam orally disintegrating tablets, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Pregnancy: Advise pregnant females that use of clonazepam orally disintegrating tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant or intend to become pregnant. Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant while taking clonazepam orally disintegrating tablets. This registry is collecting information about the safety of antiepileptic drugs during pregnancy (see PRECAUTIONS: Pregnancy).
  Nursing:  Instruct patients to inform their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients who take clonazepam orally disintegrating tablets to monitor their infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs (see PRECAUTIONS: Nursing Mothers).
Concomitant Medication : Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
Alcohol : Patients should be advised to avoid alcohol while taking clonazepam orally disintegrating tablets.
Phenylketonurics:Patients should be informed that clonazepam orally disintegrating tablets contain phenylalanine (a component of aspartame). Each orally disintegrating tablet contains 0.56 mg phenylalanine.
Drug Interactions:
Effect of Concomitant Use of Benzodiazepines and Opioids: The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation.
Effect of Clonazepam on the Pharmacokinetics of Other Drugs : Clonazepam does not appear to alter the pharmacokinetics of phenytoin, carbamazepine or phenobarbital. The effect of clonazepam on the metabolism of other drugs has not been investigated.
Effect of Other Drugs on the Pharmacokinetics of Clonazepam : Literature reports suggest that ranitidine, an agent that decreases stomach acidity, does not greatly alter clonazepam pharmacokinetics.
In a study in which the 2 mg clonazepam orally disintegrating tablet was administered with and without propantheline (an anticholinergic agent with multiple effects on the GI tract) to healthy volunteers, the AUC of clonazepam was 10% lower and the Cmax of clonazepam was 20% lower when the orally disintegrating tablet was given with propantheline compared to when it was given alone.
Fluoxetine does not affect the pharmacokinetics of clonazepam. Cytochrome P-450 inducers, such as phenytoin, carbamazepine and phenobarbital, induce clonazepam metabolism, causing an approximately 30% decrease in plasma clonazepam levels. Although clinical studies have not been performed, based on the involvement of the cytochrome P-450 3A family in clonazepam metabolism, inhibitors of this enzyme system, notably oral antifungal agents, should be used cautiously in patients receiving clonazepam.
Pharmacodynamic Interactions : The CNS-depressant action of the benzodiazepine class of drugs may be potentiated by alcohol, narcotics, barbiturates, nonbarbiturate hypnotics, antianxiety agents, the phenothiazines, thioxanthene and butyrophenone classes of antipsychotic agents, monoamine oxidase inhibitors and the tricyclic antidepressants, and by other anticonvulsant drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenicity studies have not been conducted with clonazepam.
The data currently available are not sufficient to determine the genotoxic potential of clonazepam.
In a two-generation fertility study in which clonazepam was given orally to rats at 10 and 100 mg/kg/day (low dose approximately 5 times and 24 times the maximum recommended human dose of 20 mg/day for seizure disorder and 4 mg/day for panic disorder, respectively, on a mg/m2 basis), there was a decrease in the number of pregnancies and in the number of offspring surviving until weaning.
Pregnancy:
Pregnancy Exposure Registry:
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as clonazepam orally disintegrating tablets, during pregnancy. Healthcare providers are encouraged to recommend that pregnant patients taking clonazepam orally disintegrating tablets enroll in the NAAED Pregnancy Registry by calling 1-888-233-2334 or online at http://www.aedpregnancyregistry.org/.
Risk Summary: Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
Administration of clonazepam to pregnant rabbits during the period of organogenesis resulted in developmental toxicity, including increased incidences of fetal malformations, at doses similar to or below therapeutic doses in patients (see Animal Data). Data for other benzodiazepines suggest the possibility of long-term effects on neurobehavioral and immunological function in animals following prenatal exposure to benzodiazepines at clinically relevant doses.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
 Clinical Considerations
  Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to clonazepam orally disintegrating tablets during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to clonazepam orally disintegrating tablets during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome).
 
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data: In three studies in which clonazepam orally disintegrating tablets was administered orally to pregnant rabbits at doses of 0.2, 1, 5 or 10 mg/kg/day (low dose approximately 0.2 times the maximum recommended human dose of 20 mg/day for seizure disorders and equivalent to the maximum dose of 4 mg/day for panic disorder, on a mg/m2 basis) during the period of organogenesis, a similar pattern of malformations (cleft palate, open eyelid, fused sternebrae and limb defects) was observed in a low, non-dose-related incidence in exposed litters from all dosage groups. Reductions in maternal weight gain occurred at dosages of 5 mg/kg/day or greater and reduction in embryo-fetal growth occurred in one study at a dosage of 10 mg/kg/day. No adverse maternal or embryo-fetal effects were observed in mice and rats following administration during organogenesis of oral doses up to 15 mg/kg/day or 40 mg/kg/day, respectively (4 and 20 times the maximum recommended human dose of 20 mg/day for seizure disorders and 20 and 100 times the maximum dose of 4 mg/day for panic disorder, respectively, on a mg/m2 basis).
Nursing Mothers:
Risk Summary
Clonazepam is excreted in human milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data on the effects of clonazepam on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clonazepam orally disintegrating tablets and any potential adverse effects on the breastfed infant from clonazepam orally disintegrating tablets or from the underlying maternal condition.
Clinical Considerations
 Infants exposed to clonazepam through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Pediatric Use:
Because of the possibility that adverse effects on physical or mental development could become apparent only after many years, a benefit-risk consideration of the long-term use of clonazepam orally disintegrating tablet is important in pediatric patients being treated for seizure disorder (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION).
Safety and effectiveness in pediatric patients with panic disorder below the age of 18 have not been established.
Geriatric Use:
Clinical studies of clonazepam orally disintegrating tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Because clonazepam undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Metabolites of clonazepam orally disintegrating tablets are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function. Because elderly patients are more likely to have decreased hepatic and/or renal function, care should be taken in dose selection, and it may be useful to assess hepatic and/or renal function at the time of dose selection.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of clonazepam orally disintegrating tablets and observed closely.
Adverse Reactions
The adverse experiences for clonazepam orally disintegrating tablets are provided separately for patients with seizure disorders and with panic disorder.
Seizure Disorders: The most frequently occurring side effects of clonazepam orally disintegrating tablets are referable to CNS depression. Experience in treatment of seizures has shown that drowsiness has occurred in approximately 50% of patients and ataxia in approximately 30%. In some cases, these may diminish with time; behavior problems have been noted in approximately 25% of patients. Others, uled by system, including those identified during postapproval use of clonazepam orally disintegrating tablets are:
 
Cardiovascular: Palpitations
Dermatologic: Hair loss, hirsutism, skin rash, ankle and facial edema
Gastrointestinal: Anorexia, coated tongue, constipation, diarrhea, dry mouth, encopresis, gastritis, increased appetite, nausea, sore gums
Genitourinary: Dysuria, enuresis, nocturia, urinary retention
Hematopoietic: Anemia, leukopenia, thrombocytopenia, eosinophilia
Hepatic: Hepatomegaly, transient elevations of serum transaminases and alkaline phosphatase
Musculoskeletal: Muscle weakness, pains
Miscellaneous: Dehydration, general deterioration, fever, lymphadenopathy, weight loss or gain
Neurologic: Abnormal eye movements, aphonia, choreiform movements, coma, diplopia, dysarthria, dysdiadochokinesis, ‚Äė‚Äėglassy-eyed‚Äô‚Äô appearance, headache, hemiparesis, hypotonia, nystagmus, respiratory depression, slurred speech, tremor, vertigo
Psychiatric: Confusion, depression, amnesia, hallucinations, hysteria, increased libido, insomnia, psychosis (the behavior effects are more likely to occur in patients with a history of psychiatric disturbances). The following paradoxical reactions have been observed: excitability, irritability, aggressive behavior, agitation, nervousness, hostility, anxiety, sleep disturbances, nightmares and vivid dreams
Respiratory: Chest congestion, rhinorrhea, shortness of breath, hypersecretion in upper respiratory passages
Panic Disorder: Adverse events during exposure to clonazepam orally disintegrating tablets were obtained by spontaneous report and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, CIGY dictionary terminology has been used to classify reported adverse events, except in certain cases in which redundant terms were collapsed into more meaningful terms, as noted below.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type uled. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials:
Adverse Events Associated With Discontinuation of Treatment:
Overall, the incidence of discontinuation due to adverse events was 17% in clonazepam orally disintegrating tablets compared to 9% for placebo in the combined data of two 6-to 9-week trials. The most common events (‚Č•1%) associated with discontinuation and a dropout rate twice or greater for clonazepam orally disintegrating tablets than that of placebo included the following:
Table 2: Most Common Adverse Events ( ‚Č•1%) Associated with Discontinuation of Treatment
 
Adverse Event Clonazepam Orally Disintegrating Tablets (N=574) Placebo (N=294) Somnolence 7% 1% Depression 4% 1% Dizziness 1% <1% Nervousness 1% 0% Ataxia 1% 0% Intellectual Ability Reduced 1% 0%
 
Adverse Events Occurring at an Incidence of 1% or More Among Clonazepam Orally Disintegrating Tablets-Treated Patients:  
Table 3 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred during acute therapy of panic disorder from a pool of two 6- ­to 9-week trials. Events reported in 1% or more of patients treated with clonazepam orally disintegrating tablets (doses ranging from 0.5 to 4 mg/day) and for which the incidence was greater than that in placebo-treated patients are included.
The prescriber should be aware that the figures in Table 3 cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence in the population studied.
  Table 3: Treatment-Emergent Adverse Event Incidence in 6- to 9­-Week Placebo-Controlled Clinical Trials* 
Clonazepam Maximum Daily Dose ¬† Adverse Event by Body System ¬† <1mg n=96 % ¬† 1 to <2mg n=129 % ¬† 2 to <3mg n=113 % ¬† ‚Č•3mg n=235 % ¬† All Clonazepam Orally Disintegrating Tablets Groups N=574 % ¬† Placebo N=294 % ¬† Central & Peripheral Nervous System¬† ¬† ¬† ¬† ¬† ¬† ¬† Somnolence‚Ć 26 35 50 36 37 10 Dizziness 5 5 12 8 8 4 Coordination Abnormal‚Ć 1 2 7 9 6 0 Ataxia‚Ć 2 1 8 8 5 0 Dysarthria‚Ć 0 0 4 3 2 0 Psychiatric ¬† ¬† ¬† ¬† ¬† ¬† Depression 7 6 8 8 7 1 Memory Disturbance 2 5 2 5 4 2 Nervousness 1 4 3 4 3 2 Intellectual Ability Reduced 0 2 4 3 2 0 Emotional Lability 0 1 2 2 1 1 Libido Decreased ¬† 0 1 3 1 1 0 Confusion 0 2 2 1 1 0 Respiratory System ¬† ¬† ¬† ¬† ¬† ¬† Upper Respiratory Tract Infection‚Ć ¬† 10 10 7 6 8 4 Sinusitis 4 2 8 4 4 3 Rhinitis 3 2 4 2 2 1 Coughing 2 2 4 0 2 0 Pharyngitis 1 1 3 2 2 1 Bronchitis 1 0 2 2 1 1 Gastrointestinal System ¬† ¬† ¬† ¬† ¬† ¬† Constipation‚Ć 0 1 5 3 2 2 Appetite Decreased 1 1 0 3 1 1 Abdominal Pain‚Ć 2 2 2 0 1 1 Body as a Whole ¬† ¬† ¬† ¬† ¬† ¬† Fatigue 9 6 7 7 7 4 Allergic Reaction 3 1 4 2 2 1 Musculoskeletal ¬† ¬† ¬† ¬† ¬† ¬† Myalgia 2 1 4 0 1 1 Resistance Mechanism Disorders ¬† ¬† ¬† ¬† ¬† ¬† Influenza 3 2 5 5 4 3 Urinary System ¬† ¬† ¬† ¬† ¬† ¬† Micturition Frequency 1 2 2 1 1 0 Urinary Tract Infection‚Ć 0 0 2 2 1 0 Vision Disorders ¬† ¬† ¬† ¬† ¬† ¬† Blurred Vision 1 2 3 0 1 1 Reproductive Disorders‚Ä° ¬† ¬† ¬† ¬† ¬† ¬† Female ¬† ¬† ¬† ¬† ¬† ¬† Dysmenorrhea 0 6 5 2 3 2 Colpitis 4 0 2 1 1 1 Male ¬† ¬† ¬† ¬† ¬† ¬† Ejaculation Delayed 0 0 2 2 1 0 Impotence 3 0 2 1 1 0
 
*    Events reported by at least 1% of patients treated with clonazepam orally disintegrating tablets and for which the incidence was greater than that for placebo. 
‚Ć ¬†¬† Indicates that the p-value for the dose-trend test (Cochran-Mantel-Haenszel) for adverse event incidence was ‚ȧ0.1.¬†
‡    Denominators for events in gender-specific systems are: n=240 (clonazepam), 102 (placebo) for male, and 334 (clonazepam), 192 (placebo) for female. 
 
Commonly Observed Adverse Events:
Table 4: Incidence of Most Commonly Observed Adverse Events* in Acute Therapy in Pool of 6- to 9-Week Trials
Adverse Event   Clonazepam (N=574) Placebo (N=294) Somnolence 37% 10% Depression 7% 1% Coordination Abnormal 6% 0% Ataxia 5% 0%
*¬†Treatment-emergent events for which the incidence in the clonazepam patients was ‚Č•5% and at least twice that in the placebo patients.¬†
Treatment-Emergent Depressive Symptoms:  
In the pool of two short-term placebo-controlled trials, adverse events classified under the preferred term ‚Äúdepression‚ÄĚ were reported in 7% of clonazepam orally disintegrating tablets-treated patients compared to 1% of placebo-treated patients, without any clear pattern of dose relatedness. In these same trials, adverse events classified under the preferred term ‚Äúdepression‚ÄĚ were reported as leading to discontinuation in 4% of clonazepam orally disintegrating tablets-treated patients compared to 1% of placebo-treated patients. While these findings are noteworthy, Hamilton Depression Rating Scale (HAM-D) data collected in these trials revealed a larger decline in HAM-D scores in the clonazepam group than the placebo group suggesting that clonazepam¬≠-treated patients were not experiencing a worsening or emergence of clinical depression.
Other Adverse Events Observed During the Premarketing Evaluation of Clonazepam Orally Disintegrating Tablets in Panic Disorder:  
Following is a ul of modified CIGY terms that reflect treatment-emergent adverse events reported by patients treated with clonazepam orally disintegrating tablets at multiple doses during clinical trials. All reported events are included except those already uled in Table 3 or elsewhere in labeling, those events for which a drug cause was remote, those event terms which were so general as to be uninformative, and events reported only once and which did not have a substantial probability of being acutely life-threatening. It is important to emphasize that, although the events occurred during treatment with clonazepam orally disintegrating tablets, they were not necessarily caused by it.
Events are further categorized by body system and uled in order of decreasing frequency. These adverse events were reported infrequently, which is defined as occurring in 1/100 to 1/1000 patients.
Body as a Whole: weight increase, accident, weight decrease, wound, edema, fever, shivering, abrasions, ankle edema, edema foot, edema periorbital, injury, malaise, pain, cellulitis, inflammation localized
Cardiovascular Disorders: chest pain, hypotension postural
Central and Peripheral Nervous System Disorders: migraine, paresthesia, drunkenness, feeling of enuresis, paresis, tremor, burning skin, falling, head fullness, hoarseness, hyperactivity, hypoesthesia, tongue thick, twitching
Gastrointestinal System Disorders: abdominal discomfort, gastrointestinal inflammation, stomach upset, toothache, flatulence, pyrosis, saliva increased, tooth disorder, bowel movements frequent, pain pelvic, dyspepsia, hemorrhoids
Hearing and Vestibular Disorders: vertigo, otitis, earache, motion sickness
 
Heart Rate and Rhythm Disorders: palpitation
Metabolic and Nutritional Disorders: thirst, gout
Musculoskeletal System Disorders: back pain, fracture traumatic, sprains and strains, pain leg, pain nape, cramps muscle, cramps leg, pain ankle, pain shoulder, tendinitis, arthralgia, hypertonia, lumbago, pain feet, pain jaw, pain knee, swelling knee
Platelet, Bleeding and Clotting Disorders: bleeding dermal
Psychiatric Disorders: insomnia, organic disinhibition, anxiety, depersonalization, dreaming excessive, libido loss, appetite increased, libido increased, reactions decreased, aggressive reaction, apathy, attention lack, exclient, feeling mad, hunger abnormal, illusion, nightmares, sleep disorder, suicide ideation, yawning
Reproductive Disorders, Female: breast pain, menstrual irregularity
Reproductive Disorders, Male: ejaculation decreased
Resistance Mechanism Disorders: infection mycotic, infection viral, infection streptococcal, herpes simplex infection, infectious mononucleosis, moniliasis
Respiratory System Disorders: sneezing excessive, asthmatic attack, dyspnea, nosebleed, pneumonia, pleurisy Skin and Appendages Disorders: acne flare, alopecia, xeroderma, dermatitis contact, flushing, pruritus, pustular reaction, skin burns, skin disorder
Special Senses Other, Disorders: taste loss
Urinary System Disorders: dysuria, cystitis, polyuria, urinary incontinence, bladder dysfunction, urinary retention, urinary tract bleeding, urine discoloration
Vascular (Extracardiac) Disorders: thrombophlebitis leg
Vision Disorders: eye irritation, visual disturbance, diplopia, eye twitching, styes, visual field defect, xerophthalmia
Drug Abuse And Dependence
Controlled Substance: Clonazepam is a Schedule IV controlled substance.
Abuse: Clonazepam orally disintegrating tablets is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.  Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS: Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Physical Dependence
Clonazepam orally disintegrating tablets may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS: Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam orally disintegrating tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Clonazepam orally disintegrating tablets and WARNINGS: Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used. 
Tolerance
Tolerance to clonazepam orally disintegrating tablets may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of clonazepam orally disintegrating tablets may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines. 
Following the short-term treatment of patients with panic disorder in Studies 1 and 2 (see CLINICAL PHARMACOLOGY: Clinical Trials), patients were gradually withdrawn during a 7-week downward-titration (discontinuance) period. Overall, the discontinuance period was associated with good tolerability and a very modest clinical deterioration, without evidence of a significant rebound phenomenon. However, there are not sufficient data from adequate and well-controlled long-term clonazepam studies in patients with panic disorder to accurately estimate the risks of withdrawal symptoms and dependence that may be associated with such use.
Overdosage
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, exclient, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS: Abuse, Misuse, and Addiction). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
 Consider contacting a poison center (1-800-222-1222), poisoncontrol.org, or a medical toxicologist for additional overdosage management recommendations.
Dosage And Administration
Clonazepam is available as an orally disintegrating tablet. The orally disintegrating tablet should be administered as follows: After opening the carton, peel back the foil on the buler. Do not push tablet through foil. Immediately upon opening the buler, using dry hands, remove the tablet and place it in the mouth. Tablet disintegration occurs rapidly in saliva so it can be easily swallowed with or without water.
Seizure Disorders: Adults:  The initial dose for adults with seizure disorders should not exceed 1.5 mg/day divided into three doses. Dosage may be increased in increments of 0.5 to 1 mg every 3 days until seizures are adequately controlled or until side effects preclude any further increase. Maintenance dosage must be individualized for each patient depending upon response. Maximum recommended daily dose is 20 mg.
The use of multiple anticonvulsants may result in an increase of depressant adverse effects. This should be considered before adding clonazepam orally disintegrating tablets to an existing anticonvulsant regimen.
Pediatric Patients:  Clonazepam orally disintegrating tablets are administered orally. In order to minimize drowsiness, the initial dose for infants and children (up to 10 years of age or 30 kg of body weight) should be between 0.01 and 0.03 mg/kg/day but not to exceed 0.05 mg/kg/day given in two or three divided doses. Dosage should be increased by no more than 0.25 to 0.5 mg every third day until a daily maintenance dose of 0.1 to 0.2 mg/kg of body weight has been reached, unless seizures are controlled or side effects preclude further increase. Whenever possible, the daily dose should be divided into three equal doses. If doses are not equally divided, the largest dose should be given before retiring.
Geriatric Patients:  There is no clinical trial experience with clonazepam orally disintegrating tablets in seizure disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam orally disintegrating tablets and observed closely (see PRECAUTIONS: Geriatric Use).
Panic Disorder: Adults:  The initial dose for adults with panic disorder is 0.25 mg twice daily. An increase to the target dose for most patients of 1 mg/day may be made after 3 days. The recommended dose of 1 mg/day is based on the results from a fixed dose study in which the optimal effect was seen at 1 mg/day. Higher doses of 2, 3 and 4 mg/day in that study were less effective than the 1 mg/day dose and were associated with more adverse effects. Nevertheless, it is possible that some individual patients may benefit from doses of up to a maximum dose of 4 mg/day, and in those instances, the dose may be increased in increments of 0.125 to 0.25 mg bid every 3 days until panic disorder is controlled or until side effects make further increases undesired. To reduce the inconvenience of somnolence, administration of one dose at bedtime may be desirable.
Treatment should be discontinued gradually, with a decrease of 0.125 mg bid every 3 days, until the drug is completely withdrawn.
There is no body of evidence available to answer the question of how long the patient treated with clonazepam should remain on it. Therefore, the physician who elects to use clonazepam orally disintegrating tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
Pediatric Patients:  There is no clinical trial experience with clonazepam orally disintegrating tablets in panic disorder patients under 18 years of age.
Geriatric Patients: There is no clinical trial experience with clonazepam orally disintegrating tablets in panic disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam orally disintegrating tablets and observed closely (see PRECAUTIONS: Geriatric Use).
Discontinuation or Dosage Reduction of clonazepam orally disintegrating tablets:
To reduce the risk of withdrawal reactions, increased seizure frequency, and statusepilepticus, use a gradual taper to discontinue clonazepam orally disintegrating tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence).
How Supplied
Clonazepam orally disintegrating tablets, USP are available as follows:
0.125 mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‚ÄėL 523‚Äô on one side and plain on other side, available in:
Cartons of 60 - 10 bulers  of 6 tablets each             NDC 62332-364-06
0.25 mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‚ÄėL 524‚Äô on one side and plain on other side, available in:
Cartons of 60 - 10 bulers of 6 tablets each             NDC 62332-365-06
0.5 mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‚ÄėL 525‚Äô on one side and plain on other side, available in:
Cartons of 60 - 10 bulers of 6 tablets each             NDC 62332-366-06
1 mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‚ÄėL 526‚Äô on one side and plain on other side, available in:
Cartons of 60 - 10 bulers of 6 tablets each             NDC 62332-367-06
2¬†mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‚ÄėL 527‚Äô on one side and plain on other side, available in:
Cartons of 60 - 10 bulers of 6 tablets each             NDC 62332-368-06
Store at 25¬įC (77¬įF); excursions permitted to 15¬į to 30¬įC (59¬į to 86¬įF) [see USP Controlled Room Temperature].¬†¬†
Medication Guide available at https://www.alembicusa.com/medicationguide.aspx or call 1-866-210-9797.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
  
Revised: 06/2024
Medication Guide
Clonazepam (kloe NAZ e pam) Orally Disintegrating Tablets, USP

What is the most important information I should know about clonazepam orally disintegrating tablets?
‚ÄĘ Clonazepam orally disintegrating tablet is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.
   Get emergency help right away if any of the following happens:
   o  shallow or slowed breathing
   o  breathing stops (which may lead to the heart stopping)
   o  excessive sleepiness (sedation)
Do not drive or operate heavy machinery until you know how taking clonazepam orally disintegrating tablets with opioids affects you.
‚Äʬ†Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including clonazepam orally disintegrating tablet, which can lead to overdose and serious side effects including coma and death.
   o  Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including clonazepam orally disintegrating tablets. These serious side effects may also include delirium, paranoia, suicidal thoughts oractions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
o You can develop an addiction even if you take clonazepam orally disintegrating tablets exactly as prescribed by your healthcare provider.
 o Take clonazepam orally disintegrating tablets exactly as your healthcare provider prescribed.
   o Do not share your clonazepam orally disintegrating tablets with other people.
   o Keep clonazepam orally disintegrating tablets in a safe place and away from children.
‚Äʬ† Physical dependence and withdrawal reactions. Clonazepam orally disintegrating tablets can cause physical dependence and withdrawal reactions, especially if you continue to take clonazepam orally disintegrating tablets for several days to several weeks.
   o  Do not suddenly stop taking clonazepam orally disintegrating tablets. Stopping clonazepam orally disintegrating tablets suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call  your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
  o  Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle  twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
o Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
o Do not take more clonazepam orally disintegrating tablets than prescribed or take clonazepam orally disintegrating tablets for longer than prescribed.
‚Äʬ† Clonazepam orally disintegrating tablets can make you sleepy or dizzy and can slow your thinking and motor skills. This may get better over time.
o Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam orally disintegrating tablets affects you.
o Clonazepam orally disintegrating tablets may cause problems with your coordination, especially when you are walking or picking things up.
‚Äʬ† Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clonazepam orally disintegrating tablets until you talk to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clonazepam orally disintegrating tablets may make your sleepiness or dizziness worse.
‚Äʬ† Like other antiepileptic medicines, clonazepam orally disintegrating tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
‚ÄĘ thoughts about suicide or dying ‚ÄĘ ¬†attempts to commit suicide ‚ÄĘ new or worse depression ‚ÄĘ new or worse anxiety ‚ÄĘ feeling agitated or restless ‚ÄĘ panic attacks ‚ÄĘ trouble sleeping (insomnia) ‚ÄĘ new or worse irritability ‚ÄĘ acting aggressive, being angry, or violent ‚ÄĘ acting on dangerous impulses ‚ÄĘ an extreme increase in activity and talking (mania) ‚ÄĘ other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
    o   Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
    o   Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
‚Äʬ†¬† Do not stop clonazepam orally disintegrating tablets without first talking to a healthcare provider. ¬†¬†¬† o¬†¬† Stopping clonazepam orally disintegrating tablets suddenly can cause serious problems. Stopping clonazepam orally disintegrating tablets suddenly can cause seizures that will not stop (status epilepticus).
What is clonazepam orally disintegrating tablet?
Clonazepam orally disintegrating tablet is a prescription medicine used alone or with other medicines to treat:
- certain types of seizure disorders (epilepsy) in adults and children
- panic disorder with or without fear of open spaces (agoraphobia) in adults
Clonazepam orally disintegrating tablet is a federally controlled substance (C-IV) because if contains clonazepam that can be abused or lead to dependence.
Keep  clonazepam orally disintegrating tablets in a safe place to prevent misuse and abuse. Selling orgiving away clonazepam orally disintegrating tablets may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs. It is not known if clonazepam orally disintegrating tablet is safe or effective in treating panic disorder in children younger than 18 years old.
Do not take clonazepam orally disintegrating tablet if you:
- are allergic to benzodiazepines
- have significant liver disease
- have an eye disease called acute narrow angle glaucoma
Ask your healthcare provider if you are not sure if you have any of the problems uled above. Before you take clonazepam orally disintegrating tablets, tell your healthcare provider about all your medical conditions, including if you:
- have liver or kidney problems
- have lung problems (respiratory disease)
- have or have had depression, mood problems, or suicidal thoughts or behavior
- are pregnant or plan to become pregnant.   o  Taking clonazepam orally disintegrating tablets late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).   o  Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with clonazepam orally disintegrating tablets.   o  If you become pregnant while taking clonazepam orally disintegrating tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can register by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. 
- are breastfeeding or plan to breastfeed. Clonazepam can pass into your breast milk.   o  Breastfeeding during treatment with clonazepam orally disintegrating tablets may cause your baby to have sleepiness, feeding problems, and decreased weight gain.   o  Talk to your healthcare provider about the best way to feed your baby while you take clonazepam orally disintegrating tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking clonazepam orally disintegrating tablets with certain other medicines can cause side effects or affect how well clonazepam orally disintegrating tablets or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take clonazepam orally disintegrating tablets? ‚Äʬ†¬†Take clonazepam orally disintegrating tablets exactly as your healthcare provider tells you. Clonazepam is available as a tablet or as an orally disintegrating tablet. ‚Äʬ† Do not stop taking clonazepam orally disintegrating tablets without first talking to your healthcare provider. Stopping clonazepam orally disintegrating tablets suddenly can cause serious problems.
‚Äʬ†¬†Clonazepam orally disintegrating tablets can be taken with or without water.
      o   Do not open the carton until you are ready to take clonazepam orally disintegrating tablets.
      o   After opening the carton, peel back the foil on the buler pack.
      o    Do not push the orally disintegrating tablet through the foil.
      o   After opening the buler pack, with dry hands, take the orally disintegrating tablet and place it in your mouth.
¬†¬†¬†¬†¬† o¬†¬† The orally disintegrating tablet will melt quickly. ‚Äʬ†¬†If you take too much clonazepam orally disintegrating tablets, call your healthcare provider or local Poison Control Center right away.
What should I avoid while taking clonazepam orally disintegrating tablets?
‚Äʬ†¬† Clonazepam orally disintegrating tablets can slow your thinking and motor skills. Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam orally disintegrating tablets affects you.
‚Äʬ†¬† Do not drink alcohol or take other medicines that may make you sleepy or dizzy while taking clonazepam orally disintegrating tablets until you talk to your healthcare provider. When taken with alcohol or medicines that cause sleepiness or dizziness, clonazepam orally disintegrating tablets may make your sleepiness or dizziness much worse.
What are the possible side effects of clonazepam orally disintegrating tablets?
See ‚ÄúWhat is the most important information I should know about clonazepam orally disintegrating tablets?‚ÄĚ
Clonazepam orally disintegrating tablets can also make your seizures happen more often or make them worse. Call your healthcare provider right away if your seizures get worse while taking clonazepam orally disintegrating tablets.
The most common side effects of clonazepam orally disintegrating tablets include:
‚ÄĘ drowsiness ‚ÄĘ dizziness ‚ÄĘ fatigue ‚ÄĘ problems with walking and coordination ‚ÄĘ depression ‚ÄĘ problems with memory
These are not all the possible side effects of clonazepam orally disintegrating tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Alembic Pharmaceuticals Limited at 1-866-210-9797.
  
How should I store clonazepam orally disintegrating tablets?
¬† ‚Äʬ†¬† Store clonazepam orally disintegrating tablets between 59¬įF to 86¬įF (15¬įC to 30¬įC)
¬† ‚Äʬ†¬† Keep clonazepam orally disintegrating tablets and all medicines out of the reach of children
General Information about the safe and effective use of clonazepam orally disintegrating tablets
Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use clonazepam orally disintegrating tablets for a condition for which it was not prescribed. Do not give clonazepam orally disintegrating tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about clonazepam orally disintegrating tablets that is written for health professionals.
  
For more information call Alembic Pharmaceuticals Limited at 1-866-210-9797.
  
What are the ingredients in clonazepam orally disintegrating tablets?
Active ingredient: clonazepam
Inactive ingredients: sorbitol, aspartame, sodium lauryl sulfate, crospovidone, mannitol, colloidal silicon dioxide, talc and magnesium stearate
  
This Medication Guide has been approved by the U.S. Food and Drug Administration.
  
Medication Guide available at https://www.alembicusa.com/medicationguide.aspx or call 1-866-210-9797.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921,USA
Revised: 06/2024
Package Label.principal Display Panel -0.125 Mg
NDC 62332-364-06 Clonazepam Orally Disintegrating Tablets, USP 0.125 mg Pharmacist: Dispense the accompanying Medication Guide to each patient. Rx only 60 (10 x 6) Unit Dose Tablets Alembic

Package Label.principal Display Panel -0.25 Mg
NDC 62332-365-06 Clonazepam Orally Disintegrating Tablets, USP 0.25 mg Pharmacist: Dispense the accompanying Medication Guide to each patient. Rx only 60 (10 x 6) Unit Dose Tablets Alembic

Package Label.principal Display Panel -0.5 Mg
NDC 62332-366-06 Clonazepam Orally Disintegrating Tablets, USP 0.5 mg Pharmacist: Dispense the accompanying Medication Guide to each patient. Rx only 60 (10 x 6) Unit Dose Tablets Alembic

Package Label.principal Display Panel -1 Mg
NDC 62332-367-06 Clonazepam Orally Disintegrating Tablets, USP 1 mg Pharmacist: Dispense the accompanying Medication Guide to each patient. Rx only 60 (10 x 6) Unit Dose Tablets Alembic

Package Label.principal Display Panel -2 Mg
NDC 62332-368-06 Clonazepam Orally Disintegrating Tablets, USP 2 mg Pharmacist: Dispense the accompanying Medication Guide to each patient. Rx only 60 (10 x 6) Unit Dose Tablets Alembic

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

