FIRMAGON Dailymed
Generic: degarelix is used for the treatment of Pregnancy Prostatic Neoplasms
Go PRO for all pill images
Recent Major Changes Section
Warnings and Precautions, Embryo-Fetal Toxicity ( 5.4 )02/2020
1 Indications And Usage
FIRMAGON® is indicated for treatment of patients with advanced prostate cancer.
FIRMAGON is a GnRH receptor antagonist indicated for treatment of patients with advanced prostate cancer. (1 )
2 Dosage And Administration
- FIRMAGON is for subcutaneous use only
- Starting Dosage: 240 mg given as two injections of 120 mg each (
2.1 )- Maintenance Dosage: 80 mg administered as a single injection every 28 days (
2.1 )2.1 Dosing information
FIRMAGON is administered as a subcutaneous injection in the abdominal region only at the dosages in Table 1 below.
Table 1: FIRMAGON Recommended Dosages Starting Dosage Maintenance Dosage – Administered once every 28 days
- 240 mg given as two subcutaneous injections of 120 mg at a concentration of 40 mg/mL
- The first maintenance dose should be given 28 days after the starting dose.
- 80 mg given as one subcutaneous injection at a concentration of 20 mg/mL
2.2 Reconstitution and Administration Instructions
FIRMAGON is to be administered by a healthcare professional only.
Before administering FIRMAGON read the Instructions for reconstitution and administration carefully.
As with other drugs administered by subcutaneous injection, the injection site should vary periodically. Injections should be given only in areas of the abdomen that will not be exposed to pressure, e.g., not close to waistband or belt nor close to the ribs.
FIRMAGON is supplied as a powder to be reconstituted with Sterile Water for Injection, USP.
- Starting dose (240 mg): Two single-dose vials each delivering 120 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in two prefilled syringes. Each vial is to be reconstituted with a prefilled syringe containing 3 mL of Sterile Water for Injection. 3 mL is withdrawn to deliver 120 mg degarelix at a concentration of 40 mg/mL.
- Maintenance dose (80 mg): One single-dose vial delivering 80 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in one prefilled syringe. Each vial is to be reconstituted with a prefilled syringe containing 4.2 mL of Sterile Water for Injection. 4 mL is withdrawn to deliver 80 mg degarelix at a concentration of 20 mg/mL.
Follow the instructions for reconstitution closely and read the complete instructions before performing the subcutaneous injection.
Reconstituted drug must be administered within one hour after addition of Sterile Water for Injection, USP.
Do not shake the vials.
Follow aseptic technique.
FIRMAGON 240 mg Starting Dose Kit contains:
- 2 vials containing the 120 mg FIRMAGON powder (a)
- 2 syringes containing Sterile Water for Injection, USP (b)
- 2 vial adapters (c)
- 2 25 gauge × 1 inch injection needles (d)
- 2 plunger rods (e)
 Â
FIRMAGON 80 mg Maintenance Dose Kit contains:
- 1 vial containing the 80 mg FIRMAGON powder (f)
- 1 syringe containing Sterile Water for Injection, USP (g)
- 1 vial adapter (h)
- 1 25 gauge × 1 inch injection needle (i)
- 1 plunger rod (j)
 Â
In addition the healthcare professional will need:
- gloves (k)
- alcohol pads (l)
- a clean, flat surface (m) to work on, like a table
- a sharps disposal container (n) for throwing away your used needles and syringes. See "Disposing used needles and syringes" at the end of these instructions.
The drug product must be prepared using the following instructions:
NOTE: The mixing process must be repeated for the two injections of the Starting Dose prior to injecting the product into the patient's abdomen.
Step 1: Attaching the vial adaptor to the vial IMPORTANT: DO NOT USE if there is no powder in the vial or the Sterile Water for Injection, USP is discolored.
- Thoroughly wash your hands using soap and water and put on a pair of clean gloves.
- Place all the supplies required on a clean surface.
- Check that there is powder in the FIRMAGON vial and that the Sterile Water for Injection, USP is clear and free from particles.
IMPORTANT: Do not touch the top of the vial after wiping.
- Uncap the vial containing the FIRMAGON powder (o).
- Wipe the vial rubber stopper with an alcohol pad.
IMPORTANT: Do not touch the vial adapter.
- Peel off the seal from the vial adaptor cover.
- Firmly press the vial adaptor (p) onto the vial containing the FIRMAGON powder until the adaptor snaps into place.
- Pull the vial adaptor cover off the vial.
 Â
Step 2: Assembling the syringe IMPORTANT: Do not pull the back stopper (flange) (s) off the syringe. NOTE: You will only feel light resistance screwing the plunger rod in position.
- Insert the plunger rod (q) into the prefilled syringe containing Sterile Water for Injection, USP (r) and screw the plunger rod clockwise to tighten.
 Â
Step 3: Transferring Sterile Water for Injection, USP from the syringe to the vial IMPORTANT: Do not pull off the Luer lock adaptor (u).
- Unscrew the gray syringe plug (t) attached to the Luer lock adaptor on the syringe.
IMPORTANT: Be careful not to over twist the syringe.
- Carefully twist the prefilled syringe containing Sterile Water for Injection, USP onto the vial adapter on the FIRMAGON powder vial, until it is tight.
- Press the plunger slowly to transfer all the Sterile Water for Injection, USP from the syringe to the FIRMAGON powder vial.
 Â
Step 4: Preparing the reconstituted injection
- With the syringe still attached to the vial adaptor, swirl gently until the liquid is clear with no powder or visible particles.
IMPORTANT: NOTE: If the powder adheres to the side of the vial, tilt the vial slightly. A ring of small air bubbles on the surface of the liquid is acceptable. Reconstitution time can take up to 15 min but usually takes a few minutes.
- Do not shake the vial as this will cause bubbles.
- Reconstitute just prior to administration.
 Â
Step 5: Transferring the liquid to the syringe
- Turn the vial completely upside down and pull down the plunger to withdraw all of the reconstituted liquid from the vial to the syringe.
- Tap the syringe gently with your fingers to raise air bubbles in the syringe tip.
- Press the plunger to the line marked on the syringe to expel all air bubbles.
 Â
Step 6: Preparing the syringe for injection NOTE: Reconstitute just prior to administration.
- Holding the vial adaptor detach the syringe from the vial by unscrewing the syringe from the vial adaptor.
- While holding the syringe with the tip pointing up, screw the injection needle (v) clockwise (right) onto the syringe.
 Â
Step 7: Preparing the patient
- Select one of the four available injection sites on the abdomen.
IMPORTANT:
- Do not inject in areas where the patient will be exposed to pressure, such as area around the belt of the waistband or close to the ribs.
- Vary the injection site periodically during treatment to minimize discomfort to the patient.
- Clean the injection site with an alcohol pad.
 Â
Step 8: Performing the injection
- Move the needle shield (w) away from the needle and carefully remove the needle cover (x).
IMPORTANT: If blood appears in the syringe, the product should not be injected. Discontinue the injection and discard the syringe and the needle (reconstitute a new dose for the patient).
- Pinch and elevate the skin of the abdomen.
- Insert the needle into the skin at a 45 degree angle all the way to the hub.
- Do not inject into a vein or muscle. Gently pull back the plunger to check if blood is aspirated.
- Perform a slow, deep subcutaneous injection over 30 seconds.
IMPORTANT: Do not rub the injection site after retracting the needle.
- Remove the needle and then release the skin.
 Â
Step 9: Locking the needle into the shield
- Position the needle shield approximately 45 degrees to a flat surface.
- Press down with a firm, quick motion until a distinct, audible "click" is heard.
IMPORTANT: Syringe is for single use only. Do not reuse the syringe and needle.
- Visually confirm that the needle is fully engaged under the lock (y).
 Â
Step 10: Advising the patient
- Instruct the patient not to rub or scratch the injection site.
- Inform that some patients may feel a lump at the injection site and experience redness, soreness and discomfort for a few days after the injection.
Disposing used needles and syringes
- Put used alcohol swabs, needles and syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away loose needles and syringes in the trash.
- For more information about safe sharps disposal, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
3 Dosage Forms And Strengths
For injection:
- FIRMAGON (240 mg): Two single-dose vials each delivering 120 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in two prefilled syringes.
- FIRMAGON (80 mg): One single-dose vial delivering 80 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in one prefilled syringe.
For injection:
- FIRMAGON (240 mg): Two single-dose vials each delivering 120 mg of degarelix in a lyophilized powder for reconstitution supplied with diluent in two prefilled syringes (
3 )- FIRMAGON (80 mg): One single-dose vial delivering 80 mg of degarelix in a lyophilized powder for reconstitution supplied with diluent in one prefilled syringe (
3 )
4 Contraindications
FIRMAGON is contraindicated in patients with history of severe hypersensitivity to degarelix or to any of the product components [see Warnings and Precautions (5.1)].
- Patients with history of severe hypersensitivity reactions to degarelix or to any of the product components (
4 )
5 Warnings And Precautions
- Hypersensitivity: Anaphylaxis, urticaria and angioedema have been reported. Discontinue FIRMAGON if a severe hypersensitivity reaction occurs and manage as clinically indicated (
5.1 )- QT Interval Prolongation: Androgen deprivation therapy treatment with FIRMAGON may prolong the QT interval. (
5.2 )- Embryo-Fetal Toxicity: FIRMAGON can cause fetal harm. (
5.4 ,8.1 )5.1Hypersensitivity Reactions
FIRMAGON is contraindicated in patients with history of severe hypersensitivity to degarelix or to any of the product components [see Contraindications (4)].
Hypersensitivity reactions, including anaphylaxis, urticaria and angioedema, have been reported post-marketing with FIRMAGON.
In case of a severe hypersensitivity reaction, discontinue FIRMAGON immediately if the injection has not been completed, and manage as clinically indicated. Patients with a known history of severe hypersensitivity reactions to FIRMAGON should not be re-challenged with FIRMAGON.
5.2QT Interval Prolongation
Androgen deprivation therapy may prolong the QT interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
In the randomized, active-controlled trial comparing FIRMAGON to leuprolide, periodic electro-cardiograms were performed. Seven patients, three (<1%) in the pooled degarelix group and four (2%) patients in the leuprolide 7.5 mg group, had a QTcF ≥ 500 msec. From baseline to end of study, the median change for FIRMAGON was 12.3 msec and for leuprolide was 16.7 msec.
5.3 Laboratory Testing
FIRMAGON results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after FIRMAGON may be affected. The therapeutic effect of FIRMAGON should be monitored by measuring serum concentrations of prostate-specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
5.4Embryo-Fetal Toxicity
Based on findings in animal studies, FIRMAGON can cause fetal harm and loss of pregnancy when administered to a pregnant woman. In animal developmental and reproductive toxicity studies in rats and rabbits, oral administration of degarelix during organogenesis caused embryo-fetal lethality and abortion as well as increased post-implantation loss and decreased the number of live fetuses in animals at doses less than the clinical loading dose based on body surface area. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)].
6 Adverse Reactions
Most common adverse reactions (≥10%) are injection site reactions (e.g., pain, erythema, swelling or induration), hot flashes, and increases in serum levels of transaminases and gamma-glutamyltransferase (GGT) (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Ferring at 1-888-FERRING (1-888-337-7464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
6.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
FIRMAGON was studied in a randomized, open-label trial in which patients with prostate cancer were randomized to receive FIRMAGON (subcutaneous) or leuprolide (intramuscular) monthly for 12 months [see Clinical Studies (14)].
The most common adverse reactions (≥10%) during FIRMAGON therapy are injection site reactions (e.g., pain, erythema, swelling or induration), hot flashes, and increases in serum levels of transaminases and gamma-glutamyltransferase (GGT). The majority of the adverse reactions were Grade 1 or 2, with Grade 3/4 adverse reaction incidences of 1% or less.
Adverse reactions reported in ≥ 5% of patients treated with FIRMAGON (subcutaneous) 240 mg starting dose and then 80 mg maintenance dose once every 28 days or who were treated with 7.5 mg of leuprolide (intramuscular) every 28 days are shown in Table 2.
Table 2: Adverse Reactions Reported in ≥ 5% of Patients FIRMAGON 240/80 mg (subcutaneous) N = 207 Leuprolide 7.5 mg (intramuscular) N = 201 Any adverse reaction 79% 78% Body as a whole   Injection site reactions Includes pain, erythema, swelling, induration, or nodule. 35% <1%   Weight increase 9% 12%   Chills 5% 0% Cardiovascular system   Hot flash 26% 21%   Hypertension 6% 4% Digestive system   Increases in Transaminases and GGT 10% 5%   Constipation 5% 5% Musculoskeletal system   Back pain 6% 8%   Arthralgia 5% 9% Urogenital system   Urinary tract infection 5% 9%
The following adverse reactions occurred in 1 to < 5% of patients treated with FIRMAGON:
 Body as a whole: Asthenia, fatigue, fever, night sweats Digestive system: Nausea Nervous system: Dizziness, headache, insomnia
The following adverse reactions, not already uled, occurred in ≥ 1% of patients treated in any study with FIRMAGON:
 Reproductive System: Erectile dysfunction, testicular atrophy Endocrine Disorders: Gynecomastia General: Hyperhidrosis Gastrointestinal: Diarrhea
Injection Site Reactions
The most frequently reported adverse reactions at the injection sites were pain (28%), erythema (17%), swelling (6%), induration (4%) and nodule (3%). These adverse reactions were mostly transient, of mild to moderate intensity, occurred primarily with the starting dose and led to few discontinuations (<1%). Grade 3 injection site reactions occurred in 2% or less of patients receiving FIRMAGON.
Hepatic Laboratory Abnormalities
Hepatic laboratory abnormalities were primarily Grade 1 or 2 and were generally reversible. Grade 3 hepatic laboratory abnormalities occurred in less than 1% of patients.
FIRMAGON Extension Study
The safety of FIRMAGON administered once every 28 days was evaluated further in an extension study (NCT00451958) in 385 patients who completed the above active-controlled trial. Of the 385 patients, 251 patients continued treatment with FIRMAGON and 135 patients crossed over treatment from leuprolide to FIRMAGON. The median treatment duration on the extension study was approximately 43 months (range 1 to 58 months). The most common adverse reactions reported in ≥10% of the patients were injection site reactions (e.g., pain, erythema, swelling, induration or inflammation), pyrexia, hot flush, weight loss or gain, fatigue, increases in serum levels of hepatic transaminases and GGT. One percent of patients had injection site infections including abscess. Hepatic laboratory abnormalities in the extension study included the following: Grade 1/2 elevations in hepatic transaminases occurred in 47% of patients and Grade 3 elevations occurred in 1% of patients.
6.2Immunogenicity
As with all peptides, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
Anti-Degarelix Antibodies
Anti-degarelix antibody development has been observed in 10% of patients after treatment with FIRMAGON for 1 year. There is no indication that the efficacy or safety of FIRMAGON treatment is affected by antibody formation.
6.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of FIRMAGON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Changes in bone density
Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with a GnRH agonist. It can be anticipated that long periods of medical castration in men will result in decreased bone density.
7 Drug Interactions
No drug-drug interaction studies were conducted.
Degarelix is not a substrate for the human CYP450 system. Degarelix is not an inducer or inhibitor of the CYP450 system in vitro. Therefore, clinically significant CYP450 pharmacokinetic drug-drug interactions are unlikely.
8 Use In Specific Populations
- Females and males of reproductive potential: FIRMAGON may impair fertility (
8.3 )- Patients with severe liver or kidney dysfunction have not been studied and caution is therefore warranted (
8 )8.1 Pregnancy
Risk Summary
The safety and efficacy of FIRMAGON have not been established in women.
Based on findings in animal studies and mechanism of action, FIRMAGON can cause fetal harm and loss of pregnancy when administered to a pregnant woman [see Clinical Pharmacology (12.1) ]. There are no human data on the use of FIRMAGON in pregnant women to inform the drug-associated risk. In animal developmental and reproductive toxicity studies in rats and rabbits, oral administration of degarelix during organogenesis caused embryo-fetal lethality and abortion as well as increased post-implantation loss and decreased the number of live fetuses in animals at doses less than the clinical loading dose based on body surface area (see Data ). Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
When degarelix was given to rabbits during early organogenesis at doses of 0.002 mg/kg/day (about 0.02% of the clinical loading dose based on body surface area), there was an increase in early post-implantation loss. Degarelix given to rabbits during mid and late organogenesis at doses of 0.006 mg/kg/day (about 0.05% of the clinical loading dose based on body surface area) caused embryo/fetal lethality and abortion. When degarelix was given to female rats during early organogenesis, at doses of 0.0045 mg/kg/day (about 0.036% of the clinical loading dose based on body surface area), there was an increase in early post-implantation loss. When degarelix was given to female rats during mid and late organogenesis, at doses of 0.045 mg/kg/day (about 0.36% of the clinical loading dose based on body surface area), there was an increase in the number of minor skeletal abnormalities and variants.
8.2 Lactation
The safety and efficacy of FIRMAGON have not been established in females. There are no data on the presence of degarelix in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are present in human milk and because of the potential for serious adverse reactions in a breastfed child from degarelix, a decision should be made whether to discontinue nursing or discontinue the drug taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Based on findings in animals and mechanism of action, degarelix may impair fertility in males and females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of FIRMAGON, 82% were age 65 and over, while 42% were age 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
8.6Renal Impairment
No pharmacokinetic studies in renally impaired patients have been conducted. At least 20-30% of a given dose of degarelix is excreted unchanged in the urine. A population pharmacokinetic analysis of data from the randomized study demonstrated that there is no significant effect of mild renal impairment [creatinine clearance (CrCL) 50-80 mL/min] on either the degarelix concentration or testosterone concentration. Data on patients with moderate or severe renal impairment is limited and therefore degarelix should be used with caution in patients with CrCL < 50 mL/min.
8.7Hepatic Impairment
Patients with hepatic impairment were excluded from the randomized trial.
A single dose of 1 mg degarelix administered as an intravenous infusion over 1 hour was studied in 16 non-prostate cancer patients with either mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Compared to non-prostate cancer patients with normal liver function, the exposure of degarelix decreased by 10% and 18% in patients with mild and moderate hepatic impairment, respectively. Therefore, dose adjustment is not necessary in patients with mild or moderate hepatic impairment. However, since hepatic impairment can lower degarelix exposure, it is recommended that in patients with hepatic impairment testosterone concentrations should be monitored on a monthly basis until medical castration is achieved. Once medical castration is achieved, an every-other-month testosterone monitoring approach could be considered.
Patients with severe hepatic dysfunction have not been studied and caution is therefore warranted in this group.
10 Overdosage
There have been no reports of overdose with FIRMAGON. In the case of overdose, however, discontinue FIRMAGON, treat the patient symptomatically, and institute supportive measures.
11 Description
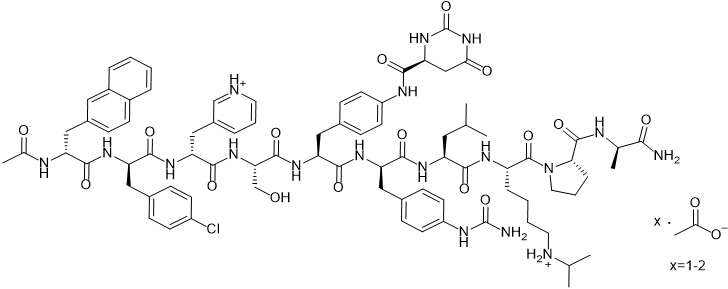
FIRMAGON is a sterile lyophilized powder for injection containing degarelix (as the acetate) and mannitol. Degarelix is a synthetic linear decapeptide amide containing seven unnatural amino acids, five of which are D-amino acids. The acetate salt of degarelix is a white to off-white amorphous powder of low density as obtained after lyophilization.
The chemical name of degarelix is D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-4-[[[(4S)-hexahydro-2,6-dioxo-4-pyrimidinyl]carbonyl]amino]-L phenylalanyl-4-[(aminocarbonyl)amino]-D-phenylalanyl-L-leucyl-N6–(1-methylethyl)-L-lysyl-L-prolyl. It has an empirical formula of C82H103N18O16Cl and a molecular weight of 1632.3 Da.
Degarelix acetate has the following structural formula:
FIRMAGON is available in two packaging configurations:
- Starting Dose: Two vial carton with each vial delivering 120 mg of degarelix (equivalent to the median value of 126 mg degarelix acetate). Each 120 mg dose contains 150 mg mannitol.
- Maintenance Dose: One-vial carton delivering 80 mg of degarelix (equivalent to the median value of 84 mg degarelix acetate). Each 80 mg dose contains 200 mg mannitol.
12 Clinical Pharmacology
12.1 Mechanism of Action
Degarelix is a GnRH receptor antagonist. It binds reversibly to the pituitary GnRH receptors, thereby reducing the release of gonadotropins and consequently testosterone.
12.2 Pharmacodynamics
A single dose of 240 mg FIRMAGON causes a decrease in the plasma concentrations of luteinizing hormone (LH) and follicle stimulating hormone (FSH), and subsequently testosterone.
FIRMAGON is effective in achieving and maintaining testosterone suppression below the castration level of 50 ng/dL.
Figure 1: Plasma Testosterone Levels from Day 0 to 364 for Degarelix 240 mg/80 mg (Median with Interquartile Ranges) 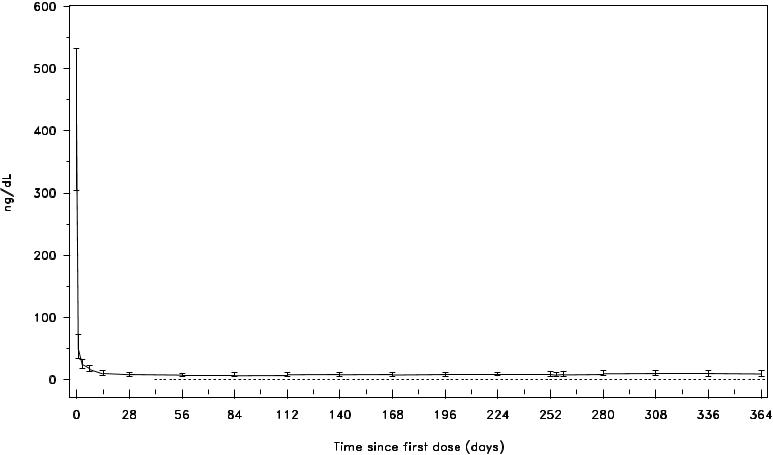
12.3 Pharmacokinetics
Absorption
FIRMAGON forms a depot upon subcutaneous administration, from which degarelix is released to the circulation. Following administration of FIRMAGON 240 mg at a product concentration of 40 mg/mL, the mean Cmax was 26.2 ng/mL (coefficient of variation, CV 83%) and the mean AUC was 1054 ng∙day/mL (CV 35%). Typically Cmax occurred within 2 days after subcutaneous administration. In prostate cancer patients at a product concentration of 40 mg/mL, the pharmacokinetics of degarelix were linear over a dose range of 120 to 240 mg. The pharmacokinetic behavior of the drug is strongly influenced by its concentration in the injection solution.
Distribution
The distribution volume of degarelix after intravenous (> 1 L/kg) or subcutaneous administration (> 1000L) indicates that degarelix is distributed throughout total body water. In vitro plasma protein binding of degarelix is estimated to be approximately 90%.
Metabolism
Degarelix is subject to peptide hydrolysis during the passage of the hepato-biliary system and is mainly excreted as peptide fragments in the feces. No quantitatively significant metabolites were detected in plasma samples after subcutaneous administration. In vitro studies have shown that degarelix is not a substrate, inducer or inhibitor of the CYP450 or p-glycoprotein transporter systems.
Excretion
Following subcutaneous administration of 240 mg FIRMAGON at a concentration of 40 mg/mL to prostate cancer patients, degarelix is eliminated in a biphasic fashion, with a median terminal half-life of approximately 53 days. The long half-life after subcutaneous administration is a consequence of a very slow release of degarelix from the FIRMAGON depot formed at the injection site(s). Approximately 20-30% of a given dose of degarelix was renally excreted, suggesting that approximately 70-80% is excreted via the hepato-biliary system in humans. Following subcutaneous administration of degarelix to prostate cancer patients the clearance is approximately 9 L/hr.
Effect of Age, Weight and Race
There was no effect of age, weight or race on the degarelix pharmacokinetic parameters or testosterone concentration.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Degarelix was administered subcutaneously to rats every 2 weeks for 2 years at doses of 2, 10 and 25 mg/kg (about 9, 45 and 120% of the recommended human loading dose on a mg/m2 basis). Long term treatment with degarelix at 25 mg/kg caused an increase in the combined incidence of benign hemangiomas plus malignant hemangiosarcomas in females.
Degarelix was administered subcutaneously to mice every 2 weeks for 2 years at doses of 2, 10 and 50 mg/kg (about 5, 22 and 120% of the recommended human loading dose (240 mg) on a mg/m2 basis). There was no statistically significant increase in tumor incidence associated with this treatment.
Degarelix did not cause genetic damage in standard in vitro assays (bacterial mutation, human lymphocyte chromosome aberration) nor in in vivo rodent bone marrow micronucleus tests.
Single degarelix doses of ≥ 1 mg/kg (about 5% of the clinical loading dose on a mg/m2 basis) caused reversible infertility in male rats. Single doses of ≥ 0.1 mg/kg (about 0.5% of the clinical loading dose on a mg/m2 basis) caused a decrease in fertility in female rats.
14 Clinical Studies
The safety and efficacy of FIRMAGON were evaluated in an open-label, multi-center, randomized, parallel-group study (NCT00295750) in patients with prostate cancer. A total of 620 patients were randomized to receive one of two FIRMAGON dosing regimens or leuprolide for one year:
a. FIRMAGON at a starting dose of 240 mg (40 mg/mL) followed by monthly doses of 80 mg (20 mg/mL) subcutaneously,b. leuprolide 7.5 mg intramuscularly monthly.c. FIRMAGON at a starting dose of 240 mg (40 mg/mL) followed by monthly doses of 160 mg (40 mg/mL) subcutaneously.
FIRMAGON is not approved for use with monthly doses of 160 mg (40 mg/mL) subcutaneously.
Serum levels of testosterone were measured at screening, on Day 0, 1, 3, 7, 14, and 28 in the first month, and then monthly until the end of the study.
The clinical trial population (n=610) across all treatment arms had an overall median age of approximately 73 (range 50 to 98). The ethnic/racial distribution was 84% white, 6% black and 10% others. Disease stage was distributed approximately as follows: 20% metastatic, 29% locally advanced (T3/T4 Nx M0 or N1 M0), 31% localized (T1 or T2 N0 M0) and 20% classified as other (including patients whose disease metastatic status could not be determined definitively - or patients with PSA relapse after primary curative therapy). In addition, the median testosterone baseline value across treatment arms was approximately 400 ng/dL.
The primary objective was to demonstrate that FIRMAGON is effective achieving and maintaining testosterone suppression to castration levels (T ≤ 50 ng/dL) during 12 months of treatment. The results are shown in Table 3.
Table 3: Medical Castration Rates (Testosterone ≤ 50 ng/dL) from Day 28 to Day 364 FIRMAGON 240/80 mg N=207 Leuprolide 7.5 mg N=201 No. of Responders 202 194 Castration Rate (95% CIs) Kaplan Meier estimates within group 97.2% (93.5; 98.8) 96.4% (92.5; 98.2)
Percentage changes in testosterone from baseline to Day 28 (median with interquartile ranges) are shown in Figure 2 and the percentages of patients who attained the medical castration of testosterone ≤ 50 ng/dL are summarized in Table 4.
Figure 2: Percentage Change in Testosterone from Baseline by Treatment Group until Day 28 (Median with Interquartile Ranges) 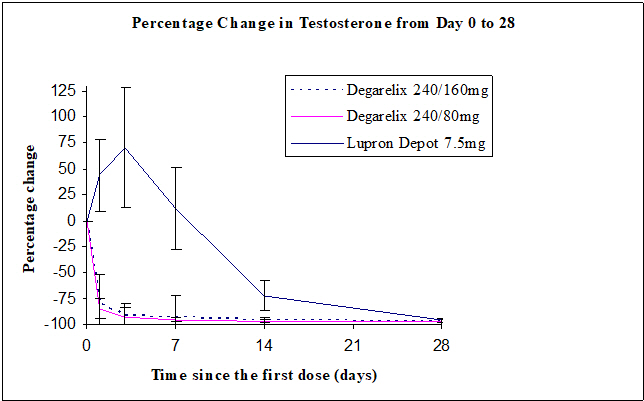
Table 4: Percentage of Patients Attaining Testosterone ≤ 50 ng/dL within the First 28 Days FIRMAGON 240/80 mg N=207 Leuprolide 7.5 mg N=201 Day 1 52% 0% Day 3 96% 0% Day 7 99% 1% Day 14 99% 18% Day 28 100% 100%
In the clinical trial, PSA levels were monitored as a secondary endpoint. PSA levels were lowered by 64% two weeks after administration of FIRMAGON, 85% after one month, 95% after three months, and remained suppressed throughout the one year of treatment. These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline is related to a clinical benefit.
16 How Supplied/storage And Handling
FIRMAGON is available as:
- NDC 55566-8403-1, Starting dose – One carton contains: Two single-dose vials each delivering 120 mg of degarelix in a white to off-white lyophilized powder for injection Two prefilled syringes each containing 3 mL of Sterile Water for Injection, USP Two vial adapters Two administration needles
- NDC 55566-8303-1, Maintenance dose – One carton contains: One single-dose vial delivering 80 mg of degarelix in a white to off-white lyophilized powder for injection One prefilled syringe containing 4.2 mL of Sterile Water for Injection, USP One vial adapter One administration needle
STORAGE AND HANDLING SECTION
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [see USP Controlled Room Temperature].
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
- Inform patients that if they have experienced severe hypersensitivity with degarelix or to any of the product components, FIRMAGON is contraindicated [see Contraindications (4)]. Instruct patients to immediately report signs of a severe hypersensitivity reaction [see Warnings and Precautions (5.1)].
QT Interval Prolongation
- Advise patients that androgen deprivation therapy treatment with FIRMAGON may prolong the QT interval. Inform patients of the signs and symptoms of QT prolongation. Advise patients to contact their healthcare provider immediately for signs or symptoms of QT prolongation [see Warnings and Precautions (5.2)].
Androgen Deprivation
- Inform patients about adverese reactions related to androgen deprivation therapy with FIRMAGON, including hot flashes, flushing of the skin, increased weight, decreased sex drive, and difficulties with erectile function [see Adverse Reactions (6.1)].
Injection Site Reactions
- Inform patients that FIRMAGON may cause redness, swelling, and itching at the injection site. Advise patients that these adverse reactions are usually mild, self limiting, and decrease within three days [see Adverse Reactions (6.1)].
Infertility
- Inform patients that FIRMAGON may cause infertility [see Use in Specific Populations (8.3)].
MANUFACTURED FOR:
FERRING PHARMACEUTICALS INC., PARSIPPANY, NJ 07054 Manufactured in Germany
02/2020
Patient Information
FIRMAGON (FIRM-uh-gahn) (degarelix for injection)
What is FIRMAGON?
FIRMAGON is a prescription medicine used in the treatment of advanced prostate cancer. It is not known if FIRMAGON is safe or effective in children.
Who should not receive FIRMAGON?
Do not receive FIRMAGON if you are allergic to degarelix or any ingredient in FIRMAGON. See the end of this leaflet for a complete ul of ingredients in FIRMAGON.
Talk to your healthcare provider before receiving FIRMAGON if you have any of these conditions.
Before receiving FIRMAGON, tell your healthcare provider about all your medical conditions, including if you:
- have any heart problems including a condition called long QT syndrome.
- have problems with blood levels such as sodium, potassium, calcium, and magnesium
- have kidney or liver problems
- are pregnant or plan to become pregnant. FIRMAGON can cause harm to your unborn baby and loss of pregnancy (miscarriage)
- are breastfeeding or plan to breastfeed. It is not known if FIRMAGON passes into your breast milk. You and your healthcare provider should decide if you will receive FIRMAGON or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a ul of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How will I receive FIRMAGON?
You will receive an injection of FIRMAGON from your healthcare provider.
- The injection site will always be in the stomach (abdominal area). The injection site will change within the stomach area each time you receive a dose of FIRMAGON.
- Two injections are given as a first dose. The following monthly doses are one injection.
- Do not rub or scratch the injection site. Make sure your injection site is free of any pressure from belts, waistbands or other types of clothing.
- Always set up an appointment for your next injection.
What are the possible side effects of FIRMAGON?
FIRMAGON can cause serious side effects, including:
- Serious allergic reactions. Get medical help right away if you get any of these symptoms:
- trouble breathing or wheezing
- severe itching
- swelling of your face, lips, mouth, or tongue
- Disorder of the heart's electrical activity. Your healthcare provider may do tests during treatment with FIRMAGON to check your heart for a condition called long QT syndrome.
The common side effects of FIRMAGON include:
- injection site pain, redness, and swelling
- hot flashes
- weight gain
- increase in some liver enzymes
Other side effects include decreased sex drive and erectile function problems.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of FIRMAGON.
Medicines are sometimes prescribed for conditions that are not mentioned in a Patient Information leaflet. Do not use FIRMAGON for a condition for which it was not prescribed. Do not give FIRMAGON to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about FIRMAGON that is written for health professionals.
What are the ingredients in FIRMAGON?
Active ingredient: degarelix (as acetate)
Inactive ingredient: mannitol
MANUFACTURED FOR:
FERRING PHARMACEUTICALS INC., PARSIPPANY, NJ 07054 Manufactured in Germany
For more information, go to www.FIRMAGON.com or call 1-888-337-7464.
This Patient Information has been approved by the U.S. Food and Drug Administration.
02/2020
Principal Display Panel - 240 Mg Kit Carton
NDC 55566-8403-1
FERRING PHARMACEUTICALS
FIRMAGON® 240 mg* (degarelix for injection)
*240 mg dose administered fromtwo vials each containing 120 mg
For single use subcutaneous injection only Discard unused portion
Kit contents: 2 vials each with 120 mg powder for injection 2 syringes with Sterile Water for Injection, USPÂ Â for reconstitution/administration 2 vial adapters 2 administration needles
Starting Dose Rx only
Principal Display Panel - 80 Mg Kit Carton
NDC 55566-8303-1
FERRING PHARMACEUTICALS
FIRMAGON® 80 mg (degarelix for injection)
For single use subcutaneous injection only Discard unused portion
Kit contents: 1 vial with 80 mg powder for injection 1 syringe with Sterile Water for Injection, USPÂ Â for reconstitution/administration 1 vial adapter 1 administration needle
Maintenance Dose (28 Days) Rx only

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

