Gabapentin (gabapentin 300 mg) Dailymed
Generic: gabapentin is used for the treatment of Bipolar Disorder Epilepsies, Partial Phobic Disorders Neuralgia, Postherpetic
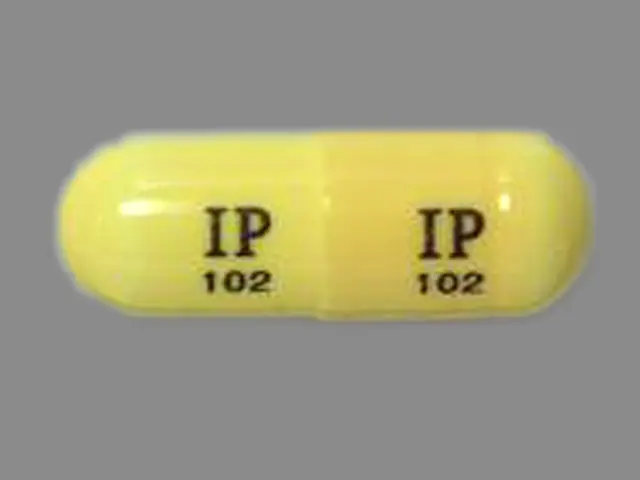
IMPRINT: IP102
SHAPE: capsule
COLOR: yellow
All Imprints
gabapentin 300 mg - ip102 capsule yellow
gabapentin 100 mg - ip101 capsule white
gabapentin 400 mg - ip103 capsule brown
Go PRO for all pill images
Description Section
Each capsule contains:
300 mg of gabapentin, USP.
Dosage & Administration Section
Dosage and Use:
See package insert
for full prescribing information
Storage And Handling Section
Store at 20 to 25 C (68 to 77 F); excursions
permitted to 15 to 30 C (59 to 86 F)) [See
USP Controlled Room Temperature].
Dispense in tight (USP), child-resistant containers.
Precautions Section
Pharmacist: Please dispense
with medication guide
provided separately
Summary Of Safety And Effectiveness
Highlights of Prescribing Information
These highlights do not include all the information needed to use gabapentin capsules safely and
effectively. See full prescribing information for gabapentin capsules.
GABAPENTIN capsules, USP for oral use
Initial U.S. Approval: 1993
FULL PRESCRIBING INFORMATION:  CONTENTS
Indications & Usage Section
INDICATIONS AND USAGE
Gabapentin capsules, USP, are indicated for:
    -management of postherpeticneuralgia in adults
    -Adjunctive therapy in the treatment of partial onset seizures, with and without secondary
generalization, in adults and pediatric patients 3 years and older with epilepsy
Dosage & Administration Section
DOSAGE AND ADMINISTRATION
Gabapentin capsules, USP are given orally with or without food. Gabapentin capsules, USP should be
swallowed whole with plenty of water.
Dosage Forms & Strengths Section
DOSAGE FORMS AND STRENGTHS
Capsules:
- 100 mg; white-white, opague hard gelatin capsules printed with "IP 101 " on both cap and body.
- 300 mg: buff-buff, opague hard gelatin capsules printed with "IP 102" on both cap and body.
- 400 mg: light caramel-light caramel, opague hard gelatin capsules printed with "IP 103" on both cap and body
Contraindications Section
CONTRAINDICATIONS
Gabapentin capsules, USP are contraindicated in patients who have demonstrated hypersensitivity to
the drug or its ingredients.
Warnings And Precautions Section
WARNINGS AND PRECAUTIONS
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Multiorgan Hypersensitivity
Drug Reaction with with Eosinophilia and Systemic Symptoms
Adverse Reactions Section
ADVERSE REACTIONS
The following severe adverse reactions are discussed in greater detail in other sections: Drug Reaction
with Eosiniphilia and Systemic Syndrome (DRESS) Multiorgan
Drug Interactions Section
DRUG INTERACTIONS
Other Antiepileptic Drugs Gabapentin is not appreciably metabolized nor does it interfere
with the metabolism of commonly co-administered antiepileptic drugs
Use In Specific Populations Section
USE IN SPECIFIC POPULATIONS
Pregnanacy - Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women.
Drug Abuse And Dependence Section
DRUG ABUSE AND DEPENDENCE
Controlled Substance - Gabapentin is not a scheduled drug.
Overdosage Section
OVERDOSAGE
A lethal dose of gabapentin was not identified in mice and rats receiving single oral doses as high
as 8000 mg / kg.
Description Section
DESCRIPTION
The active ingredient in gabapentin capsules, USP is gabapentin which has the chemical name
1-(aminoethyl) cyclohexaneacetic acid.
Clinical Pharmacology Section
CLINICAL PHARMACOLOGY
Mechanism of Action - The precise mechanisms by which gabapentin produces its analgesic
and antiepileptic actions are unknown.
Nonclinical Toxicology Section
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility - Gabapentin was adminstered orally to
mice and rats in 2-year carcinogenicity studies.
Clinical Studies Section
CLINICAL STUDIES
Postherpetic Neuralgia  Gabapentin was evaluated for the management of postherpetic neuralgia
(PHN) in two randomized, double-blind, placebo-controlled multicenter studies.
How Supplied Section
HOW SUPPLIED/STORAGE AND HANDLING
Gabapentin capsules, USP
Patient Medication Information Section
PATIENT COUNSELING INFORMATION
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site