Gentamicin Sulfate Dailymed
Generic: gentamicin sulfate is used for the treatment of Bone Diseases, Infectious Central Nervous System Infections Endocarditis Endocarditis, Bacterial Escherichia coli Infections Hypersensitivity Klebsiella Infections Proteus Infections Pseudomonas Infections Respiratory Tract Infections Staphylococcal Infections Peritonitis, Tuberculous Urinary Tract Infections Eye Infections, Bacterial Serratia Infections Skin Diseases, Bacterial Soft Tissue Infections Sepsis
Go PRO for all pill images
Sterile
Rx only
Description
Gentamicin sulfate is a water-soluble antibiotic of the aminoglycoside group.
Gentamicin Sulfate Ophthalmic Solution is a sterile, aqueous solution for ophthalmic use.
Each mL contains: Active: Gentamicin Sulfate USP (equivalent to 3 mg gentamicin base) Preservative: Benzalkonium Chloride Inactives: Disodium Phosphate, Monosodium Phosphate, and Sodium Chloride. The pH range is from 6.8 to 7.3.
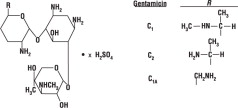
Gentamicin is obtained from cultures of Micromonospora purpurea. It is a mixture of the sulfate salts of gentamicin C1, C2, and C1A. All three components appear to have similar antimicrobial activities. Gentamicin sulfate occurs as a white powder and is soluble in water and insoluble in alcohol. The structural formula is as follows:
Clinical Pharmacology
Microbiology
Gentamicin sulfate is active in vitro against many strains of the following microorganisms:
 Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Serratia marcescens.
Indications And Usage
Gentamicin Sulfate Sterile Ophthalmic Solution is indicated in the topical treatment of ocular bacterial infections, including conjunctivitis, keratitis, keratoconjunctivitis, corneal ulcers, blepharitis, blepharoconjunctivitis, acute meibomianitis, and dacryocystitis caused by susceptible strains of the following microorganisms:
 Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Serratia marcescens.
Contraindications
Gentamicin Sulfate Sterile Ophthalmic Solution is contraindicated in patients with known hypersensitivity to any of the components.
Warnings
NOT FOR INJECTION INTO THE EYE. Gentamicin Sulfate Ophthalmic Solution is not for injection. It should never be injected subconjunctivally, nor should it be directly introduced into the anterior chamber of the eye.
Precautions
General
Prolonged use of topical antibiotics may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to gentamicin may also develop. If purulent discharge, inflammation or pain becomes aggravated, the patient should discontinue use of the medication and consult a physician.
If irritation or hypersensitivity to any component of the drug develops, the patient should discontinue use of this preparation and appropriate therapy should be instituted.
Information for patients
To avoid contamination, do not touch tip of container to the eye, eyelid or any surface.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no published carcinogenicity or impairment of fertility studies on gentamicin. Aminoglycoside antibiotics have been found to be non mutagenic.
Pregnancy
Pregnancy Category C
Gentamicin has been shown to depress body weights, kidney weights and median glomerular counts in newborn rats when administered systemically to pregnant rats in daily doses approximately 500 times the maximum recommended ophthalmic human dose. There are no adequate and well-controlled studies in pregnant women. Gentamicin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pediatric Use
Safety and effectiveness in neonates have not been established.
Adverse Reactions
Bacterial and fungal corneal ulcers have developed during treatment with gentamicin ophthalmic preparations.
The most frequently reported adverse reactions are ocular burning and irritation upon drug instillation, non-specific conjunctivitis, conjunctival epithelial defects and conjunctival hyperemia.
Other adverse reactions which have occurred rarely are allergic reactions, thrombocytopenic purpura and hallucinations.
Dosage And Administration
Gentamicin Sulfate sterile ophthalmic solution; Instill one or two drops into the affected eye(s) every four hours. In severe infections, dosage may be increased to as much as two drops once every hour.
How Supplied
Gentamicin Sulfate ophthalmic solution - Sterile, 5-mL plastic dropper bottle, box of one. (NDC 17478-283-10)
STORAGE AND HANDLING SECTION
STORAGE: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Avoid exposure to excessive heat.
Akorn
Manufactured by: Akorn, Inc. Lake Forest, IL 60045
GK00N
Rev. 06/16
Package Label.principal Display Panel
Principal Display Panel Text for Container Label:
NDC 17478-283-10
Gentamicin
Sulfate Ophthalmic
Solution, USP
0.3%
(equivalent to 3 mg
gentamicin per mL)
Sterile
Rx only 5 mL

Package Label.principal Display Panel
Principal Display Panel Text for Carton Label:
NDC 17478-283-10
Gentamicin
Sulfate
Ophthalmic
Solution, USP
0.3%
(equivalent to 3 mg
gentamicin per mL)
5 mL
Sterile
Rx only Akorn Logo
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


