Trijardy XR (empagliflozin 12.5 mg linagliptin 2.5 mg metformin hydrochloride 1000 mg) Dailymed
Generic: empagliflozin, linagliptin, metformin hydrochloride is used for the treatment of Diabetes Mellitus, Type 2 Cardiovascular Diseases Kidney Diseases Acidosis Liver Diseases Diabetic Ketoacidosis Renal Insufficiency
Boxed Warning
Warning: Lactic Acidosis
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. Symptoms included malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Laboratory abnormalities included elevated blood lactate levels, anion gap acidosis, increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. (
5.1 ) - Risk factors include renal impairment, concomitant use of certain drugs, age ≥65 years old, radiological studies with contrast, surgery and other procedures, hypoxic states, excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information. (
5.1 ) - If lactic acidosis is suspected, discontinue TRIJARDY XR and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended. (
5.1 )
Go PRO for all pill images
Warning: Lactic Acidosis
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7), and Use in Specific Populations (8.6, 8.7)].
If metformin-associated lactic acidosis is suspected, immediately discontinue TRIJARDY XR and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)].
WARNING: LACTIC ACIDOSIS
See full prescribing information for complete boxed warning.
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. Symptoms included malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Laboratory abnormalities included elevated blood lactate levels, anion gap acidosis, increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. (
5.1 )- Risk factors include renal impairment, concomitant use of certain drugs, age ≥65 years old, radiological studies with contrast, surgery and other procedures, hypoxic states, excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information. (
5.1 )- If lactic acidosis is suspected, discontinue TRIJARDY XR and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended. (
5.1 )
Recent Major Changes Section
Warnings and Precautions ( 5.5 ,5.7 )10/2022
1 Indications And Usage
TRIJARDY XR is a combination of empagliflozin, linagliptin, and metformin hydrochloride (HCl) indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease [see Clinical Studies (14)].
TRIJARDY XR is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin hydrochloride (HCl), a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease. (1 )
Limitations of Use
- Not recommended in patients with type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients (
1 )- Has not been studied in patients with a history of pancreatitis (
1 )
Limitations of Use
TRIJARDY XR is not recommended in patients with type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients [see Warnings and Precautions (5.3)].
TRIJARDY XR has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at an increased risk for the development of pancreatitis while using TRIJARDY XR [see Warnings and Precautions (5.2)].
2 Dosage And Administration
- Assess renal function before initiating and as clinically indicated (
2.1 )- Individualize the starting dose based on the patient's current regimen and renal function (
2.2 ,2.3 )- Initiation is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2, due to the metformin component (
2.3 )- The maximum recommended dose of TRIJARDY XR is 25 mg empagliflozin, 5 mg linagliptin and 2000 mg metformin HCl (
2.2 )- Take once daily with a meal in the morning (
2.2 )- Swallow whole; do not split, crush, dissolve, or chew (
2.2 )- TRIJARDY XR may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures (
2.4 )2.1 Prior to Initiation of TRIJARDY XR
- Assess renal function before initiating TRIJARDY XR and as clinically indicated [see Warnings and Precautions (5.1, 5.4)].
- In patients with volume depletion, correct this condition before initiating TRIJARDY XR [see Warnings and Precautions (5.4), Use in Specific Populations (8.5, 8.6)].
2.2 Recommended Dosage and Administration
- Individualize the starting dose of TRIJARDY XR based on the patient's current regimen:
- In patients on metformin HCl, with or without linagliptin, switch to TRIJARDY XR containing a similar total daily dose of metformin HCl and a total daily dose of empagliflozin 10 mg and linagliptin 5 mg;
- In patients on metformin HCl and any regimen containing empagliflozin, with or without linagliptin, switch to TRIJARDY XR containing a similar total daily dose of metformin HCl, the same total daily dose of empagliflozin and linagliptin 5 mg.
- Monitor effectiveness and tolerability, and adjust dosing as appropriate, not to exceed the maximum recommended daily dose of empagliflozin 25 mg, linagliptin 5 mg and metformin HCl 2000 mg.
- Take TRIJARDY XR orally, once daily with a meal in the morning.
- Take TRIJARDY XR 10 mg/5 mg/1000 mg or TRIJARDY XR 25 mg/5 mg/1000 mg as a single tablet once daily.
- Take TRIJARDY XR 5 mg/2.5 mg/1000 mg or TRIJARDY XR 12.5 mg/2.5 mg/1000 mg as two tablets together once daily.
- Swallow TRIJARDY XR tablets whole. Do not split, crush, dissolve, or chew.
2.3 Dosage Recommendations in Patients with Renal Impairment
- Initiation of TRIJARDY XR is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2, due to the metformin component.
- TRIJARDY XR is contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 or in patients on dialysis [see Contraindications (4), Warnings and Precautions (5.1, 5.4) and Use in Specific Populations (8.6)].
2.4 Discontinuation for Iodinated Contrast Imaging Procedures
Discontinue TRIJARDY XR at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR less than 60 mL/min/1.73 m2; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart TRIJARDY XR if renal function is stable [see Warnings and Precautions (5.1)].
3 Dosage Forms And Strengths
TRIJARDY XR Tablets:
Empagliflozin Strength Linagliptin Strength Metformin HCl Extended-Release Strength Color/Shape Tablet Markings 5 mg 2.5 mg 1000 mg grey, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "395" on the top line and "5/2.5" on the bottom line 10 mg 5 mg 1000 mg tan, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "380" on the top line and "10/5" on the bottom line 12.5 mg 2.5 mg 1000 mg red, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "385" on the top line and "12.5/2.5" on the bottom line 25 mg 5 mg 1000 mg brown, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "390" on the top line and "25/5" on the bottom line
Tablets:
- 5 mg empagliflozin/2.5 mg linagliptin/1000 mg metformin HCl extended-release (
3 )- 10 mg empagliflozin/5 mg linagliptin/1000 mg metformin HCl extended-release (
3 )- 12.5 mg empagliflozin/2.5 mg linagliptin/1000 mg metformin HCl extended-release (
3 )- 25 mg empagliflozin/5 mg linagliptin/1000 mg metformin HCl extended-release (
3 )
4 Contraindications
TRIJARDY XR is contraindicated in patients with:
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end-stage renal disease, or dialysis [see Warnings and Precautions (5.1, 5.4) and Use in Specific Populations (8.6)].
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis [see Warnings and Precautions (5.1)].
- Hypersensitivity to empagliflozin, linagliptin, metformin or any of the excipients in TRIJARDY XR, reactions such as anaphylaxis, angioedema, exfoliative skin conditions, urticaria, or bronchial hyperreactivity have occurred [see Warnings and Precautions (5.9) and Adverse Reactions (6)].
- Severe renal impairment (eGFR below 30 mL/min/1.73 m2), end-stage renal disease, or on dialysis (
4 ,5.1 ,5.4 )- Metabolic acidosis, including diabetic ketoacidosis (
1 ,4 ,5.1 )- Hypersensitivity to empagliflozin, linagliptin, metformin, or any of the excipients in TRIJARDY XR (
4 ,5.9 )
5 Warnings And Precautions
- Lactic Acidosis: See
boxed warning (5.1 )- Pancreatitis: There have been reports of acute pancreatitis, including fatal pancreatitis. If pancreatitis is suspected, promptly discontinue TRIJARDY XR. (
5.2 )- Ketoacidosis: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level. If suspected, discontinue TRIJARDY XR, evaluate and treat promptly. Before initiating TRIJARDY XR, consider risk factors for ketoacidosis. Patients on TRIJARDY XR may require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis. (
5.3 )- Volume Depletion: Before initiating TRIJARDY XR, assess volume status and renal function in patients with impaired renal function, elderly patients, or patients on loop diuretics. Monitor for signs and symptoms during therapy. (
5.4 ,6.1 )- Urosepsis and Pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated (
5.5 )- Hypoglycemia: Consider lowering the dose of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating TRIJARDY XR. (
5.6 )- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (
5.7 )- Genital Mycotic Infections: Monitor and treat as appropriate (
5.8 )- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema, and exfoliative skin conditions) have occurred with empagliflozin and linagliptin. If hypersensitivity reactions occur, discontinue TRIJARDY XR, treat promptly, and monitor until signs and symptoms resolve. (
5.9 )- Vitamin B12 Deficiency: Metformin may lower vitamin B12 levels. Measure hematologic parameters annually and vitamin B12 at 2 to 3 year intervals and manage any abnormalities. (
5.10 )- Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue TRIJARDY XR if appropriate. (
5.11 )- Bullous Pemphigoid: There have been reports of bullous pemphigoid requiring hospitalization. Tell patients to report development of bulers or erosions. If bullous pemphigoid is suspected, discontinue TRIJARDY XR. (
5.12 )- Heart Failure: Heart failure has been observed with two other members of the DPP-4 inhibitor class. Consider risks and benefits of TRIJARDY XR in patients who have known risk factors for heart failure. Monitor for signs and symptoms. (
5.13 )5.1 Lactic Acidosis
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypothermia, hypotension, and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate:pyruvate ratio; metformin plasma levels generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels, which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of TRIJARDY XR. In TRIJARDY XR-treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin is dialyzable, with a clearance of up to 170 mL/minute under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and if these symptoms occur instruct them to discontinue TRIJARDY XR and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
Renal Impairment: The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment. The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]:
- Before initiating TRIJARDY XR, obtain an estimated glomerular filtration rate (eGFR).
- TRIJARDY XR is contraindicated in patients with an eGFR below 30 mL/min/1.73 m2 [see Contraindications (4)].
- Obtain an eGFR at least annually in all patients taking TRIJARDY XR. In patients at increased risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
Drug Interactions: The concomitant use of TRIJARDY XR with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance or increase metformin accumulation [see Drug Interactions (7)]. Therefore, consider more frequent monitoring of patients.
Age 65 or Greater: The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients [see Use in Specific Populations (8.5)].
Radiological Studies with Contrast: Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop TRIJARDY XR at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR less than 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart TRIJARDY XR if renal function is stable.
Surgery and Other Procedures: Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension and renal impairment. TRIJARDY XR should be temporarily discontinued while patients have restricted food and fluid intake.
Hypoxic States: Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur, discontinue TRIJARDY XR.
Excessive Alcohol Intake: Alcohol potentiates the effect of metformin on lactate metabolism and this may increase the risk of metformin-associated lactic acidosis. Warn patients against excessive alcohol intake while receiving TRIJARDY XR.
Hepatic Impairment: Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of TRIJARDY XR in patients with clinical or laboratory evidence of hepatic disease.
5.2 Pancreatitis
Acute pancreatitis, including fatal pancreatitis, has been reported in patients treated with linagliptin. In the CARMELINA trial [see Clinical Studies (14)], acute pancreatitis was reported in 9 (0.3%) patients treated with linagliptin and in 5 (0.1%) patients treated with placebo. Two patients treated with linagliptin in the CARMELINA trial had acute pancreatitis with a fatal outcome. There have been postmarketing reports of acute pancreatitis, including fatal pancreatitis, in patients treated with linagliptin.
Take careful notice of potential signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue TRIJARDY XR and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using TRIJARDY XR.
5.3 Ketoacidosis
Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization have been identified in clinical trials and postmarketing surveillance in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors, including empagliflozin. Fatal cases of ketoacidosis have been reported in patients taking empagliflozin. In placebo-controlled trials of patients with type 1 diabetes, the risk of ketoacidosis was increased in patients who received SGLT2 inhibitors compared to patients who received placebo. TRIJARDY XR is not indicated for the treatment of patients with type 1 diabetes mellitus [see Indications and Usage (1)].
Patients treated with TRIJARDY XR who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with TRIJARDY XR may be present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is suspected, TRIJARDY XR should be discontinued, patient should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, fluid and carbohydrate replacement.
In many of the postmarketing reports, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized and institution of treatment was delayed because presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. In some but not all cases, factors predisposing to ketoacidosis such as insulin dose reduction, acute febrile illness, reduced caloric intake, surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
Before initiating TRIJARDY XR, consider factors in the patient history that may predispose to ketoacidosis including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse.
For patients who undergo scheduled surgery, consider temporarily discontinuing TRIJARDY XR for at least 3 days prior to surgery [see Clinical Pharmacology (12.2, 12.3)].
Consider monitoring for ketoacidosis and temporarily discontinuing TRIJARDY XR in other clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or post-surgery). Ensure risk factors for ketoacidosis are resolved prior to restarting TRIJARDY XR.
Educate patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue TRIJARDY XR and seek medical attention immediately if signs and symptoms occur.
5.4 Volume Depletion
Empagliflozin can cause intravascular volume depletion which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)]. There have been post-marketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including empagliflozin. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating TRIJARDY XR in patients with one or more of these characteristics, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating TRIJARDY XR. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
5.5 Urosepsis and Pyelonephritis
There have been reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving SGLT2 inhibitors, including empagliflozin. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6)].
5.6 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues are known to cause hypoglycemia. The risk of hypoglycemia is increased when TRIJARDY XR is used in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin. Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with TRIJARDY XR.
5.7 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in patients with diabetes mellitus receiving SGLT2 inhibitors, including empagliflozin. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with TRIJARDY XR presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue TRIJARDY XR, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.8 Genital Mycotic Infections
Empagliflozin increases the risk for genital mycotic infections [see Adverse Reactions (6.1)]. Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop genital mycotic infections. Monitor and treat as appropriate.
5.9 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with linagliptin. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions. Onset of these reactions occurred predominantly within the first 3 months after initiation of treatment with linagliptin, with some reports occurring after the first dose.
Angioedema has also been reported with other dipeptidyl peptidase-4 (DPP-4) inhibitors. Use caution in a patient with a history of angioedema to another DPP-4 inhibitor because it is unknown whether such patients will be predisposed to angioedema with TRIJARDY XR.
There have been postmarketing reports of serious hypersensitivity reactions (e.g., angioedema) in patients treated with empagliflozin.
If a hypersensitivity reaction occurs, discontinue TRIJARDY XR, treat promptly per standard of care, and monitor until signs and symptoms resolve. TRIJARDY XR is contraindicated in patients with hypersensitivity to linagliptin, empagliflozin or any of the excipients in TRIJARDY XR [see Contraindications (4)].
5.10 Vitamin B Deficiency
In metformin clinical trials of 29-week duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels was observed in approximately 7% of metformin-treated patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, may be associated with anemia but appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. Measure hematologic parameters on an annual basis and vitamin B12 at 2 to 3 year intervals in patients on TRIJARDY XR and manage any abnormalities [see Adverse Reactions (6.1)].
5.11 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.12 Bullous Pemphigoid
Bullous pemphigoid was reported in 7 (0.2%) patients treated with linagliptin compared to none in patients treated with placebo in the CARMELINA trial [see Clinical Studies (14)], and 3 of these patients were hospitalized due to bullous pemphigoid. Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of bulers or erosions while receiving TRIJARDY XR. If bullous pemphigoid is suspected, TRIJARDY XR should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
5.13 Heart Failure
An association between DPP-4 inhibitor treatment and heart failure has been observed in cardiovascular outcomes trials for two other members of the DPP-4 inhibitor class. These trials evaluated patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease.
Consider the risks and benefits of TRIJARDY XR prior to initiating treatment in patients at risk for heart failure, such as those with a prior history of heart failure and a history of renal impairment, and observe these patients for signs and symptoms of heart failure during therapy. Advise patients of the characteristic symptoms of heart failure and to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of TRIJARDY XR.
6 Adverse Reactions
The following important adverse reactions are described below and elsewhere in the labeling:
- Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)]
- Pancreatitis [see Warnings and Precautions (5.2)]
- Ketoacidosis [see Warnings and Precautions (5.3)]
- Volume Depletion [see Warnings and Precautions (5.4)]
- Urosepsis and Pyelonephritis [see Warnings and Precautions (5.5)]
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues [see Warnings and Precautions (5.6)]
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene) [see Warnings and Precautions (5.7)]
- Genital Mycotic Infections [see Warnings and Precautions (5.8)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.9)]
- Vitamin B12 Deficiency [see Warnings and Precautions (5.10)]
- Severe and Disabling Arthralgia [see Warnings and Precautions (5.11)]
- Bullous Pemphigoid [see Warnings and Precautions (5.12)]
- Heart Failure [see Warnings and Precautions (5.13)]
Most common adverse reactions (5% or greater incidence) were upper respiratory tract infection, urinary tract infection, nasopharyngitis, diarrhea, constipation, headache, and gastroenteritis. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Empagliflozin, Linagliptin and Metformin
The safety of concomitantly administered empagliflozin (daily dose 10 mg or 25 mg), linagliptin (daily dose 5 mg) and metformin has been evaluated in a total of 686 patients with type 2 diabetes treated for up to 52 weeks in an active-controlled clinical trial. The most common adverse reactions are shown in Table 1.
Table 1 Adverse Reactions Reported in ≥5% of Patients Treated with Empagliflozin, Linagliptin, and Metformin in an Active-Controlled Clinical Trial of 52 Weeks Adverse Reactions Empagliflozin 10 mg + Linagliptin 5 mg + Metformin (%) n=136 Empagliflozin 25 mg + Linagliptin 5 mg + Metformin (%) n=137 aPredefined grouping, including, but not limited to, urinary tract infection, asymptomatic bacteriuria, cystitis Upper respiratory tract infection 10.3 8.0 Urinary tract infectiona 9.6 10.2 Nasopharyngitis 8.1 5.8 Diarrhea 6.6 2.2 Constipation 5.1 5.8 Headache 5.1 5.1 Gastroenteritis 2.9 5.8
Hypoglycemia
The incidence of hypoglycemia (defined as plasma or capillary glucose of less than 54 mg/dL) was 0.7% in patients receiving empagliflozin 10 mg/linagliptin 5 mg/metformin and 0.7% in patients receiving empagliflozin 25 mg/linagliptin 5 mg/metformin. Events of severe hypoglycemia (requiring assistance regardless of blood glucose) did not occur in this trial.
Empagliflozin
Adverse reactions that occurred in ≥2% of patients receiving empagliflozin and more commonly than in patients given placebo included (10 mg, 25 mg, and placebo): urinary tract infection (9.3%, 7.6%, and 7.6%), female genital mycotic infections (5.4%, 6.4%, and 1.5%), upper respiratory tract infection (3.1%, 4.0%, and 3.8%), increased urination (3.4%, 3.2%, and 1.0%), dyslipidemia (3.9%, 2.9%, and 3.4%), arthralgia (2.4%, 2.3%, and 2.2%), male genital mycotic infections (3.1%, 1.6%, and 0.4%), and nausea (2.3%, 1.1%, and 1.4%).
Thirst (including polydipsia) was reported in 0%, 1.7%, and 1.5% for placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
Empagliflozin causes an osmotic diuresis, which may lead to intravascular volume contraction and adverse reactions related to volume depletion. Events related to volume depletion (hypotension and syncope) were reported in 3 patients (1.1%) treated with empagliflozin, linagliptin and metformin combination therapy.
Linagliptin
Adverse reactions reported in ≥2% of patients treated with linagliptin 5 mg and more commonly than in patients treated with placebo, included: nasopharyngitis (7.0% and 6.1%), diarrhea (3.3% and 3.0%), and cough (2.1% and 1.4%).
Other adverse reactions reported in clinical studies with treatment of linagliptin monotherapy were hypersensitivity (e.g., urticaria, angioedema, localized skin exfoliation, or bronchial hyperreactivity) and myalgia.
In the clinical trial program, pancreatitis was reported in 15.2 cases per 10,000 patient-year exposure while being treated with linagliptin, compared with 3.7 cases per 10,000 patient-year exposure while being treated with comparator (placebo and active comparator, sulfonylurea). Three additional cases of pancreatitis were reported following the last administered dose of linagliptin.
Metformin
The most common (>5%) established adverse reactions due to initiation of metformin therapy are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache.
In a 24-week clinical trial in which extended-release metformin or placebo was added to glyburide therapy, the most common (>5% and greater than placebo) adverse reactions in the combined treatment group were hypoglycemia (13.7% vs 4.9%), diarrhea (12.5% vs 5.6%), and nausea (6.7% vs 4.2%).
Laboratory Tests
Empagliflozin
Linagliptin
Metformin
6.2 Postmarketing Experience
Additional adverse reactions have been identified during postapproval use of linagliptin, empagliflozin, or metformin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Acute Pancreatitis, including Fatal Pancreatitis [see Indications and Usage (1)]
- Ketoacidosis
- Urosepsis and Pyelonephritis
- Necrotizing Fasciitis of the Perineum (Fournier's gangrene)
- Hypersensitivity Reactions including Anaphylaxis, Angioedema, and Exfoliative Skin Conditions
- Severe and Disabling Arthralgia
- Bullous Pemphigoid
- Acute Kidney Injury
- Skin Reactions (e.g., rash, urticaria)
- Mouth Ulceration, Stomatitis
- Cholestatic, hepatocellular, and mixed hepatocellular liver injury
- Rhabdomyolysis
7 Drug Interactions
Table 2 Clinically Relevant Interactions with TRIJARDY XR Carbonic Anhydrase Inhibitors Clinical Impact Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently causes a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with TRIJARDY XR may increase the risk of lactic acidosis. Intervention Consider more frequent monitoring of these patients. Drugs that Reduce Metformin Clearance Clinical Impact Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors such as ranolazine, vandetanib, dolutegravir, and cimetidine) could increase systemic exposure to metformin and may increase the risk for lactic acidosis [see Clinical Pharmacology (12.3)]. Intervention Consider the benefits and risks of concomitant use. Alcohol Clinical Impact Alcohol is known to potentiate the effect of metformin on lactate metabolism. Intervention Warn patients against excessive alcohol intake while receiving TRIJARDY XR. Diuretics Clinical Impact Coadministration of empagliflozin with diuretics resulted in increased urine volume and frequency of voids, which might enhance the potential for volume depletion. Intervention Before initiating TRIJARDY XR, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating TRIJARDY XR. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy. Insulin or Insulin Secretagogues Clinical Impact The risk of hypoglycemia is increased when TRIJARDY XR is used in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin. Intervention Coadministration of TRIJARDY XR with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. Drugs Affecting Glycemic Control Clinical Impact Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. Intervention When such drugs are administered to a patient receiving TRIJARDY XR, the patient should be closely observed to maintain adequate glycemic control. When such drugs are withdrawn from a patient receiving TRIJARDY XR, the patient should be observed closely for hypoglycemia. Lithium Clinical Impact Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. Intervention Monitor serum lithium concentration more frequently during TRIJARDY XR initiation and dosage changes. Inducers of P-glycoprotein or CYP3A4 Enzymes Clinical Impact Rifampin decreased linagliptin exposure, suggesting that the efficacy of linagliptin may be reduced when administered in combination with a strong P-gp or CYP3A4 inducer. Intervention Use of alternative treatments is strongly recommended when linagliptin is to be administered with a strong P-gp or CYP3A4 inducer. Positive Urine Glucose Test Clinical Impact SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Intervention Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. Interference with 1,5-anhydroglucitol (1,5-AG) Assay Clinical Impact Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Intervention Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control.
- Carbonic Anhydrase Inhibitors: May increase risk of lactic acidosis. Consider more frequent monitoring. (
7 )- Drugs that Reduce Metformin Clearance: May increase risk of lactic acidosis. Consider benefits and risks of concomitant use. (
7 )- See full prescribing information for additional drug interactions and information on interference of TRIJARDY XR with laboratory tests. (
7 )
8 Use In Specific Populations
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters (
8.1 )- Lactation: Not recommended when breastfeeding (
8.2 )- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (
8.3 )- Geriatric Patients: Higher incidence of adverse reactions related to volume depletion and reduced renal function (
5.1 ,5.4 ,8.5 )- Renal Impairment: Higher incidence of adverse reactions related to reduced renal function (
2.3 ,5.1 ,5.4 ,8.6 )- Hepatic Impairment: Avoid use in patients with hepatic impairment (
8.7 )8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects from empagliflozin, TRIJARDY XR is not recommended during the second and third trimesters of pregnancy.
The limited available data with TRIJARDY XR, linagliptin, or empagliflozin in pregnant women are not sufficient to determine a drug-associated risk for major birth defects and miscarriage. Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
In animal studies, empagliflozin, a component of TRIJARDY XR, resulted in adverse renal changes in rats when administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy. Doses approximately 13-times the maximum clinical dose caused renal pelvic and tubule dilatations that were reversible. No adverse developmental effects were observed when linagliptin or metformin were administered to pregnant rats or rabbits (see Data).
The estimated background risk of major birth defects is 6% to 10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20% to 25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk: Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from postmarketing studies have not reported a clear association with metformin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was used during pregnancy. However, these studies cannot definitely establish the absence of any metformin-associated risk because of methodological limitations, including small sample size and inconsistent comparator groups.
Animal Data
8.2 Lactation
Risk Summary
There is limited information regarding the presence of TRIJARDY XR, or its components (empagliflozin, linagliptin, or metformin) in human milk, the effects on the breastfed infant, or the effects on milk production. Limited published studies report that metformin is present in human milk (see Data). Empagliflozin and linagliptin are present in rat milk (see Data). Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney.
Because of the potential for serious adverse reactions in a breastfed infant, including the potential for empagliflozin to affect postnatal renal development, advise patients that use of TRIJARDY XR is not recommended while breastfeeding.
Data
Published clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin during lactation because of small sample size and limited adverse event data collected in infants.
Empagliflozin was present at a low level in rat fetal tissues after a single oral dose to the dams at gestation day 18. In rat milk, the mean milk to plasma ratio ranged from 0.634 to 5, and was greater than one from 2 to 24 hours post-dose. The mean maximal milk to plasma ratio of 5 occurred at 8 hours post-dose, suggesting accumulation of empagliflozin in the milk. Juvenile rats directly exposed to empagliflozin showed a risk to the developing kidney (renal pelvic and tubular dilatations) during maturation.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with metformin may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of TRIJARDY XR have not been established in pediatric patients.
8.5 Geriatric Use
Assess renal function more frequently in TRIJARDY XR-treated geriatric patients because there is a greater risk of empagliflozin-associated intravascular volume contraction and symptomatic hypotension in geriatric patients and there is a greater risk of metformin-associated lactic acidosis in geriatric patients [see Warnings and Precautions (5.1, 5.4)].
The recommended dosage for the metformin component of TRIJARDY XR in geriatric patients should usually start at the lower end of the dosage range.
Of the 273 patients treated with the combination of empagliflozin, linagliptin, and metformin hydrochloride to improve glycemic control in adults with type 2 diabetes mellitus, 58 were 65 years of age and older, while 8 were 75 years of age and older. Clinical studies of TRIJARDY XR did not include sufficient numbers of geriatric patients to determine whether they respond differently from younger adult patients.
Empagliflozin
In empagliflozin type 2 diabetes studies, 2721 empagliflozin-treated patients were 65 years of age and older and 491 patients were 75 years of age and older. In these studies, volume depletion-related adverse reactions occurred in 2.1%, 2.3%, and 4.4% of patients 75 years of age and older in the placebo, empagliflozin 10 mg, and empagliflozin 25 mg once daily groups, respectively; and urinary tract infections occurred in 10.5%, 15.7%, and 15.1% of patients 75 years of age and older in the placebo, empagliflozin 10 mg, and empagliflozin 25 mg once daily groups, respectively.
Linagliptin
In linagliptin studies, 1085 linagliptin-treated patients were 65 years of age and older and 131 patients were 75 years of age and older. In these linagliptin studies, no overall differences in safety or effectiveness of linagliptin were observed between geriatric patients and younger adult patients.
Metformin
Clinical studies of metformin did not include sufficient numbers of geriatric patients to determine whether they respond differently from younger adult patients.
8.6 Renal Impairment
TRIJARDY XR should not be initiated in patients with an eGFR less than 45 mL/min/1.73 m2 due to the metformin component and is contraindicated in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end-stage renal disease, or dialysis.
Empagliflozin
The glucose lowering benefit of empagliflozin 25 mg decreased in patients with worsening renal function. The risks of renal impairment [see Warnings and Precautions (5.4)], volume depletion adverse reactions and urinary tract infection-related adverse reactions increased with worsening renal function.
Metformin
Metformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment [see Warnings and Precautions (5.1)].
8.7 Hepatic Impairment
Use of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. TRIJARDY XR is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.1)].
10 Overdosage
In the event of an overdose with TRIJARDY XR, contact the Poison Control Center.
Overdose of metformin HCl has occurred, including ingestion of amounts greater than 50 grams. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions (5.1)]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
Removal of empagliflozin by hemodialysis has not been studied, and removal of linagliptin by hemodialysis or peritoneal dialysis is unlikely.
11 Description
TRIJARDY XR tablets for oral use contain: empagliflozin, linagliptin, and metformin hydrochloride.
Empagliflozin
Empagliflozin is an inhibitor of the sodium-glucose co-transporter 2 (SGLT2).
The chemical name of empagliflozin is D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-, (1S).
The molecular formula is C23H27ClO7 and the molecular weight is 450.91. The structural formula is:
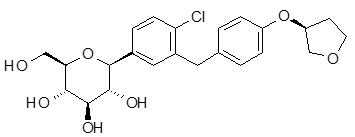
Empagliflozin is a white to yellowish, non-hygroscopic powder. It is very slightly soluble in water, sparingly soluble in methanol, slightly soluble in ethanol and acetonitrile, soluble in 50% acetonitrile/water, and practically insoluble in toluene.
Linagliptin
Linagliptin is an inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme.
The chemical name of linagliptin is 1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-
The molecular formula is C25H28N8O2 and the molecular weight is 472.54. The structural formula is:
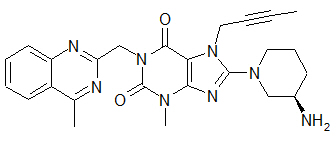
Linagliptin is a white to yellowish, not or only slightly hygroscopic solid substance. It is very slightly soluble in water. Linagliptin is soluble in methanol, sparingly soluble in ethanol, very slightly soluble in isopropanol, and very slightly soluble in acetone.
Metformin hydrochloride
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a biguanide. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5∙HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
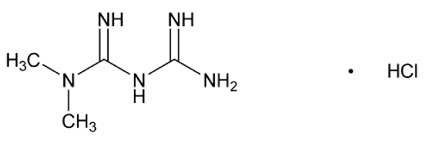
TRIJARDY XR
Each film coated tablet of TRIJARDY XR consists of an extended-release metformin hydrochloride core tablet that is coated with the immediate-release drug substances: empagliflozin and linagliptin.
TRIJARDY XR tablets for oral administration are available in four strengths containing:
- 5 mg empagliflozin/2.5 mg linagliptin/1000 mg metformin hydrochloride extended-release
- 10 mg empagliflozin/5 mg linagliptin/1000 mg metformin hydrochloride extended-release
- 12.5 mg empagliflozin/2.5 mg linagliptin/1000 mg metformin hydrochloride extended-release
- 25 mg empagliflozin/5 mg linagliptin/1000 mg metformin hydrochloride extended-release
Each film-coated tablet of TRIJARDY XR contains the following inactive ingredients: Tablet Core: polyethylene oxide, hypromellose, and magnesium stearate. Film Coatings and Printing Ink: hydroxypropyl cellulose, hypromellose, talc, titanium dioxide, arginine, polyethylene glycol, carnauba wax, purified water, shellac glaze, n-butyl alcohol, propylene glycol, ammonium hydroxide, isopropyl alcohol, ferrosoferric oxide and ferric oxide yellow (5 mg/2.5 mg/1000 mg and 25 mg/5 mg/1000 mg), ferric oxide yellow and ferric oxide red (10 mg/5 mg/1000 mg), and ferrosoferric oxide and ferric oxide red (12.5 mg/2.5 mg /1000 mg).
12 Clinical Pharmacology
12.1 Mechanism of Action
TRIJARDY XR
TRIJARDY XR contains: empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin, a biguanide.
Empagliflozin
Empagliflozin is an inhibitor of the sodium-glucose co-transporter 2 (SGLT2), the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Linagliptin
Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output.
Metformin HCl
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may decrease.
12.2 Pharmacodynamics
Empagliflozin
Urinary Glucose Excretion
In patients with type 2 diabetes, urinary glucose excretion increased immediately following a dose of empagliflozin and was maintained at the end of a 4-week treatment period averaging at approximately 64 grams per day with 10 mg empagliflozin and 78 grams per day with 25 mg empagliflozin once daily. Data from single oral doses of empagliflozin in healthy subjects indicate that, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days for the 10 mg and 25 mg doses.
Urinary Volume
In a 5-day study, mean 24-hour urine volume increase from baseline was 341 mL on Day 1 and 135 mL on Day 5 of empagliflozin 25 mg once daily treatment.
Cardiac Electrophysiology
In a randomized, placebo-controlled, active-comparator, crossover study, 30 healthy subjects were administered a single oral dose of empagliflozin 25 mg, empagliflozin 200 mg (8 times the maximum recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either 25 mg or 200 mg empagliflozin.
Linagliptin
Linagliptin binds to DPP-4 in a reversible manner and increases the concentrations of incretin hormones. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion, thus resulting in a better regulation of the glucose homeostasis. Linagliptin binds selectively to DPP-4 and selectively inhibits DPP-4, but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
Cardiac Electrophysiology
In a randomized, placebo-controlled, active-comparator, 4-way crossover study, 36 healthy subjects were administered a single oral dose of linagliptin 5 mg, linagliptin 100 mg (20 times the recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either the recommended dose of 5 mg or the 100-mg dose. At the 100-mg dose, peak linagliptin plasma concentrations were approximately 38-fold higher than the peak concentrations following a 5-mg dose.
12.3 Pharmacokinetics
Administration of TRIJARDY XR with food resulted in no change in overall exposure of empagliflozin or linagliptin. For metformin extended-release, high-fat meals increased systemic exposure (as measured by area-under-the-curve [AUC]) by approximately 70% relative to fasting, while Cmax is not affected. Meals prolonged Tmax by approximately 3 hours.
Empagliflozin
Absorption
The pharmacokinetics of empagliflozin has been characterized in healthy volunteers and patients with type 2 diabetes and no clinically relevant differences were noted between the two populations. After oral administration, peak plasma concentrations of empagliflozin were reached at 1.5 hours post-dose. Thereafter, plasma concentrations declined in a biphasic manner with a rapid distribution phase and a relatively slow terminal phase. The steady-state mean plasma AUC and Cmax were 1870 nmol∙h/L and 259 nmol/L, respectively, with 10 mg empagliflozin once daily treatment, and 4740 nmol∙h/L and 687 nmol/L, respectively, with 25 mg empagliflozin once daily treatment. Systemic exposure of empagliflozin increased in a dose-proportional manner in the therapeutic dose range. The single-dose and steady-state pharmacokinetic parameters of empagliflozin were similar, suggesting linear pharmacokinetics with respect to time.
Distribution
The apparent steady-state volume of distribution was estimated to be 73.8 L based on a population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, the red blood cell partitioning was approximately 36.8% and plasma protein binding was 86.2%.
Elimination
The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 h and apparent oral clearance was 10.6 L/h based on the population pharmacokinetic analysis. Following once-daily dosing, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state, which was consistent with empagliflozin half-life.
Linagliptin
Absorption
The absolute bioavailability of linagliptin is approximately 30%. A high-fat meal reduced Cmax by 15% and increased AUC by 4%; this effect is not clinically relevant. Linagliptin may be administered with or without food.
Distribution
The mean apparent volume of distribution at steady-state following a single intravenous dose of linagliptin 5 mg to healthy subjects is approximately 1110 L, indicating that linagliptin extensively distributes to the tissues. Plasma protein binding of linagliptin is concentration-dependent, decreasing from about 99% at 1 nmol/L to 75% to 89% at ≥30 nmol/L, reflecting saturation of binding to DPP-4 with increasing concentration of linagliptin. At high concentrations, where DPP-4 is fully saturated, 70% to 80% of linagliptin remains bound to plasma proteins and 20% to 30% is unbound in plasma. Plasma binding is not altered in patients with renal or hepatic impairment.
Elimination
Linagliptin has a terminal half-life of about 200 hours at steady-state, though the accumulation half-life is about 11 hours. Renal clearance at steady-state was approximately 70 mL/min.
Metformin HCl
Absorption
Following a single oral dose of 1000 mg (2 × 500 mg tablets) metformin HCl extended-release after a meal, the time to reach maximum plasma metformin concentration (Tmax) is achieved at approximately 7 to 8 hours. In both single- and multiple-dose studies in healthy subjects, once daily 1000 mg (2 × 500 mg tablets) dosing provides equivalent systemic exposure, as measured by AUC, and up to 35% higher Cmax of metformin relative to the immediate-release given as 500 mg twice daily.
Single oral doses of metformin HCl extended-release from 500 mg to 2500 mg resulted in less than proportional increase in both AUC and Cmax. Low-fat and high-fat meals increased the systemic exposure (as measured by AUC) from metformin extended-release tablets by about 38% and 73%, respectively, relative to fasting. Both meals prolonged metformin Tmax by approximately 3 hours but Cmax, was not affected.
Distribution
The apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin HCl tablets 850 mg averaged 654±358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time.
Elimination
Metformin has a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
Hepatic Impairment
Effects of Age, Body Mass Index, Gender, and Race
Drug Interactions
Pharmacokinetic drug interaction studies with TRIJARDY XR have not been performed; however, such studies have been conducted with the individual components of TRIJARDY XR (empagliflozin, linagliptin, and metformin HCl).
Empagliflozin
Linagliptin
Metformin HCl
Table 5 Effect of Coadministered Drug on Plasma Metformin Systemic Exposure Coadministered Drug Dosing of Coadministered Drug* Dosing of Metformin HCl* Geometric Mean Ratio (ratio with/without coadministered drug) No effect=1.0 *All metformin and coadministered drugs were given as single doses †AUC = AUC(INF) ≠Metformin HCl extended-release tablets 500 mg ‡Ratio of arithmetic means **At steady-state with topiramate 100 mg every 12 hours and metformin 500 mg every 12 hours; AUC = AUC(0-12 hours) AUC†Cmax Glyburide 5 mg 500 mg≠metformin 0.98‡ 0.99‡ Furosemide 40 mg 850 mg metformin 1.09‡ 1.22‡ Nifedipine 10 mg 850 mg metformin 1.16 1.21 Propranolol 40 mg 850 mg metformin 0.90 0.94 Ibuprofen 400 mg 850 mg metformin 1.05‡ 1.07‡ Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination [see Drug Interactions (7)]. Cimetidine 400 mg 850 mg metformin 1.40 1.61 Carbonic anhydrase inhibitors may cause metabolic acidosis [see Drug Interactions (7)]. Topiramate** 100 mg 500 mg metformin 1.25 1.17
Table 6 Effect of Metformin on Coadministered Drug Systemic Exposure Coadministered Drug Dosing of Coadministered Drug* Dosing of Metformin HCl* Geometric Mean Ratio (ratio with/without metformin) No effect=1.0 *All metformin and coadministered drugs were given as single doses †AUC = AUC(INF) unless otherwise noted §AUC(0-24 hours) reported ‡Ratio of arithmetic means, p-value of difference <0.05 ¶Ratio of arithmetic means AUC†Cmax Glyburide 5 mg 500 mg§ glyburide 0.78‡ 0.63‡ Furosemide 40 mg 850 mg furosemide 0.87‡ 0.69‡ Nifedipine 10 mg 850 mg nifedipine 1.10§ 1.08 Propranolol 40 mg 850 mg propranolol 1.01§ 0.94 Ibuprofen 400 mg 850 mg ibuprofen 0.97¶ 1.01¶ Cimetidine 400 mg 850 mg cimetidine 0.95§ 1.01
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
TRIJARDY XR
No carcinogenicity, mutagenicity, or impairment of fertility studies have been conducted with the combination of empagliflozin, linagliptin, and metformin HCl.
Empagliflozin
Carcinogenesis was evaluated in 2-year studies conducted in CD-1 mice and Wistar rats. Empagliflozin did not increase the incidence of tumors in female rats dosed at 100, 300, or 700 mg/kg/day (up to 72 times the exposure from the maximum clinical dose of 25 mg). In male rats, hemangiomas of the mesenteric lymph node were increased significantly at 700 mg/kg/day or approximately 42 times the exposure from a 25 mg clinical dose. Empagliflozin did not increase the incidence of tumors in female mice dosed at 100, 300, or 1000 mg/kg/day (up to 62 times the exposure from a 25 mg clinical dose). Renal tubule adenomas and carcinomas were observed in male mice at 1000 mg/kg/day, which is approximately 45 times the exposure of the maximum clinical dose of 25 mg. These tumors may be associated with a metabolic pathway predominantly present in the male mouse kidney.
Empagliflozin was not mutagenic or clastogenic with or without metabolic activation in the in vitro Ames bacterial mutagenicity assay, the in vitro L5178Y tk+/- mouse lymphoma cell assay, and an in vivo micronucleus assay in rats.
Empagliflozin had no effects on mating, fertility or early embryonic development in treated male or female rats, up to the high dose of 700 mg/kg/day (approximately 155 times the 25 mg clinical dose in males and females, respectively).
Linagliptin
Linagliptin did not increase the incidence of tumors in male and female rats in a 2-year study at doses of 6, 18, and 60 mg/kg. The highest dose of 60 mg/kg is approximately 418 times the clinical dose of 5 mg/day based on AUC exposure. Linagliptin did not increase the incidence of tumors in mice in a 2-year study at doses up to 80 mg/kg (males) and 25 mg/kg (females), or approximately 35 and 270 times the clinical dose based on AUC exposure. Higher doses of linagliptin in female mice (80 mg/kg) increased the incidence of lymphoma at approximately 215 times the clinical dose based on AUC exposure.
Linagliptin was not mutagenic or clastogenic with or without metabolic activation in the Ames bacterial mutagenicity assay, a chromosomal aberration test in human lymphocytes, and an in vivo micronucleus assay.
In fertility studies in rats, linagliptin had no adverse effects on early embryonic development, mating, fertility, or bearing live young up to the highest dose of 240 mg/kg (approximately 943 times the clinical dose based on AUC exposure).
Metformin HCl
Long-term carcinogenicity studies have been performed in Sprague Dawley rats at doses of 150, 300, and 450 mg/kg/day in males and 150, 450, 900, and 1200 mg/kg/day in females. These doses are approximately 2, 4, and 8 times in males, and 3, 7, 12, and 16 times in females of the maximum recommended human daily dose of 2000 mg/kg/day based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female rats. A carcinogenicity study was also performed in Tg.AC transgenic mice at doses of up to 2000 mg/kg/day applied dermally. No evidence of carcinogenicity was observed in male or female mice.
Genotoxicity assessments in the Ames test, gene mutation test (mouse lymphoma cells), chromosomal aberrations test (human lymphocytes) and in vivo mouse micronucleus tests were negative.
Fertility of male or female rats was not affected by metformin when administered at doses up to 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose based on body surface area comparisons.
14 Clinical Studies
Empagliflozin and Linagliptin Add-on Combination Therapy with Metformin for Glycemic Control
A total of 686 patients with type 2 diabetes participated in a double-blind, active-controlled study to evaluate the efficacy and safety of empagliflozin 10 mg or 25 mg in combination with linagliptin 5 mg, compared to the individual components.
Patients with type 2 diabetes inadequately controlled on at least 1500 mg of metformin per day entered a single-blind placebo run-in period for 2 weeks. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7% and 10.5% were randomized 1:1:1:1:1 to one of 5 active-treatment arms of empagliflozin 10 mg or 25 mg, linagliptin 5 mg, or linagliptin 5 mg in combination with 10 mg or 25 mg empagliflozin as a fixed dose combination tablet.
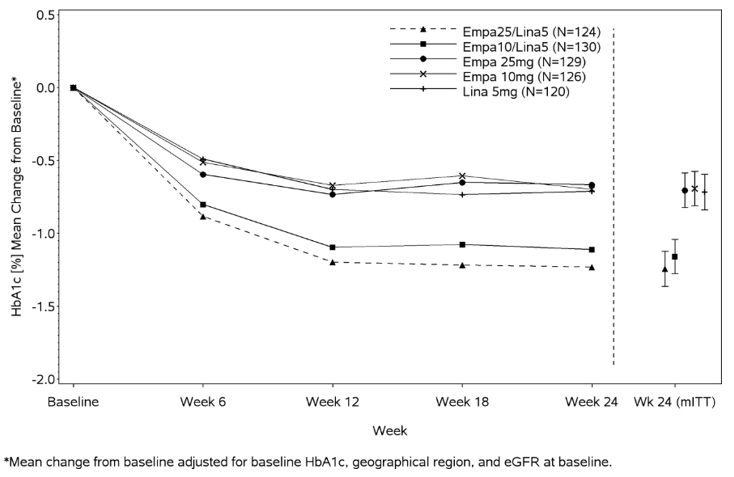
At Week 24, empagliflozin 10 mg or 25 mg used in combination with linagliptin 5 mg provided statistically significant improvement in HbA1c (p-value <0.0001) and FPG (p-value <0.001) compared to the individual components in patients who had been inadequately controlled on metformin (see Table 7, Figure 3). Treatment with empagliflozin 10 mg or 25 mg used in combination with linagliptin 5 mg also resulted in a statistically significant reduction in body weight compared to linagliptin 5 mg (p-value <0.0001). There was no statistically significant difference compared to empagliflozin alone.
Table 7 Glycemic Parameters at 24 Weeks in a Study Comparing Empagliflozin in Combination with Linagliptin to the Individual Components as Add-on Therapy in Patients Inadequately Controlled on Metformin Empagliflozin 10 mg/Linagliptin 5 mg Empagliflozin 25 mg/Linagliptin 5 mg Empagliflozin 10 mg Empagliflozin 25 mg Linagliptin 5 mg aFull analysis population (observed case) using MMRM. MMRM model included treatment, renal function, region, visit, visit by treatment interaction, and baseline HbA1c. bPatients with HbA1c above 7% at baseline: empagliflozin 25 mg/linagliptin 5 mg, n=123; empagliflozin 10 mg/linagliptin 5 mg, n=128; empagliflozin 25 mg, n=132; empagliflozin 10 mg, n=125; linagliptin 5 mg, n=119. Non-completers were considered failures (NCF). cFull analysis population using last observation carried forward. ANCOVA model included treatment, renal function, region, baseline weight, and baseline HbA1c. dp<0.001 for FPG; p<0.0001 for HbA1c and body weight HbA1c (%)   Number of patients n=135 n=133 n=137 n=139 n=128   Baseline (mean) 8.0 7.9 8.0 8.0 8.0   Change from baseline (adjusted mean) -1.1 -1.2 -0.7 -0.6 -0.7   Comparison vs empagliflozin 25 mg or 10 mg (adjusted mean) (95% CI)a -0.4 (-0.6, -0.2)d -0.6 (-0.7, -0.4)d -- -- --   Comparison vs linagliptin 5 mg (adjusted mean) (95% CI)a -0.4 (-0.6, -0.2)d -0.5 (-0.7, -0.3)d -- -- --   Patients [n (%)] achieving HbA1c <7%b 74 (58) 76 (62) 35 (28) 43 (33) 43 (36) FPG (mg/dL)   Number of patients n=133 n=131 n=136 n=137 n=125   Baseline (mean) 157 155 162 160 156   Change from baseline (adjusted mean) -33 -36 -21 -21 -13   Comparison vs empagliflozin 25 mg or 10 mg (adjusted mean) (95% CI)a -12 (-18, -5)d -15 (-22, -9)d -- -- --   Comparison vs linagliptin 5 mg (adjusted mean) (95% CI)a -20 (-27, -13)d -23 (-29, -16)d -- -- -- Body Weight   Number of patients n=135 n=134 n=137 n=140 n=128   Baseline (mean) in kg 87 85 86 88 85   % change from baseline (adjusted mean) -3.1 -3.4 -3.0 -3.5 -0.7   Comparison vs empagliflozin 25 mg or 10 mg (adjusted mean) (95% CI)c 0.0 (-0.9, 0.8) 0.1 (-0.8, 0.9) -- -- --   Comparison vs linagliptin 5 mg (adjusted mean) (95% CI)c -2.4 (-3.3, -1.5)d -2.7 (-3.6, -1.8)d -- -- --
Figure 3 Adjusted Mean HbA1c Change at Each Time Point (Completers) and at Week 24 (mITT population)
Empagliflozin Cardiovascular Outcome Study in Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease
Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease. The effect of empagliflozin on cardiovascular risk in adult patients with type 2 diabetes and established, stable, atherosclerotic cardiovascular disease is presented below.
The EMPA-REG OUTCOME study, a multicenter, multinational, randomized, double-blind parallel group trial compared the risk of experiencing a major adverse cardiovascular event (MACE) between empagliflozin and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and atherosclerotic cardiovascular disease. Coadministered antidiabetic medications were to be kept stable for the first 12 weeks of the trial. Thereafter, antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
A total of 7020 patients were treated (empagliflozin 10 mg = 2345; empagliflozin 25 mg = 2342; placebo = 2333) and followed for a median of 3.1 years. Approximately 72% of the study population was Caucasian, 22% was Asian, and 5% was Black. The mean age was 63 years and approximately 72% were male.
All patients in the study had inadequately controlled type 2 diabetes mellitus at baseline (HbA1c greater than or equal to 7%). The mean HbA1c at baseline was 8.1% and 57% of participants had diabetes for more than 10 years. Approximately 31%, 22% and 20% reported a past history of neuropathy, retinopathy and nephropathy to investigators, respectively and the mean eGFR was 74 mL/min/1.73 m2. At baseline, patients were treated with one (~30%) or more (~70%) antidiabetic medications including metformin (74%), insulin (48%), sulfonylurea (43%) and dipeptidyl peptidase-4 inhibitor (11%).
All patients had established atherosclerotic cardiovascular disease at baseline including one (82%) or more (18%) of the following: a documented history of coronary artery disease (76%), stroke (23%) or peripheral artery disease (21%). At baseline, the mean systolic blood pressure was 136 mmHg, the mean diastolic blood pressure was 76 mmHg, the mean LDL was 86 mg/dL, the mean HDL was 44 mg/dL, and the mean urinary albumin to creatinine ratio (UACR) was 175 mg/g. At baseline, approximately 81% of patients were treated with renin angiotensin system inhibitors, 65% with beta-blockers, 43% with diuretics, 77% with statins, and 86% with antiplatelet agents (mostly aspirin).
The primary endpoint in EMPA-REG OUTCOME was the time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event was defined as occurrence of either a cardiovascular death or a non-fatal myocardial infarction (MI) or a non-fatal stroke. The statistical analysis plan had pre-specified that the 10 and 25 mg doses would be combined. A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE and superiority on MACE if non-inferiority was demonstrated. Type-1 error was controlled across multiples tests using a hierarchical testing strategy.
Empagliflozin significantly reduced the risk of first occurrence of primary composite endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (HR: 0.86; 95% CI: 0.74, 0.99). The treatment effect was due to a significant reduction in the risk of cardiovascular death in subjects randomized to empagliflozin (HR: 0.62; 95% CI: 0.49, 0.77), with no change in the risk of non-fatal myocardial infarction or non-fatal stroke (see Table 8 and Figures 4 and 5). Results for the 10 mg and 25 mg empagliflozin doses were consistent with results for the combined dose groups.
Table 8 Treatment Effect for the Primary Composite Endpoint and its Componentsa Placebo N=2333 Empagliflozin N=4687 Hazard ratio vs placebo (95% CI) aTreated set (patients who had received at least one dose of study drug) bp–value for superiority (2–sided) 0.04 cTotal number of events Composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke (time to first occurrence)b 282 (12.1%) 490 (10.5%) 0.86 (0.74, 0.99) Non-fatal myocardial infarctionc 121 (5.2%) 213 (4.5%) 0.87 (0.70, 1.09) Non-fatal strokec 60 (2.6%) 150 (3.2%) 1.24 (0.92, 1.67) Cardiovascular deathc 137 (5.9%) 172 (3.7%) 0.62 (0.49, 0.77)
Figure 4 Estimated Cumulative Incidence of First MACE
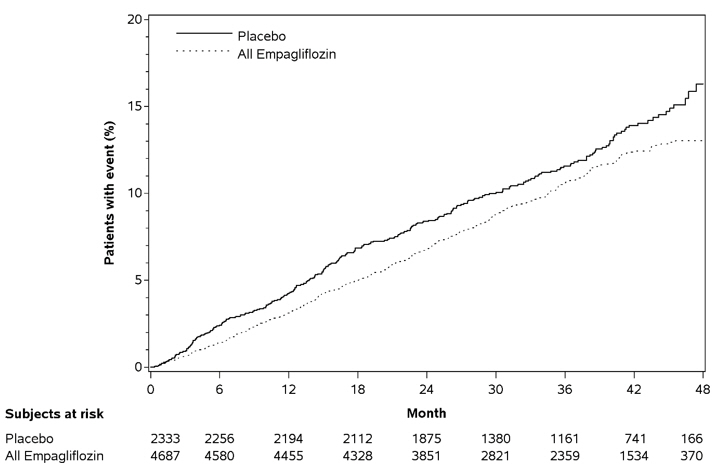
Figure 5 Estimated Cumulative Incidence of Cardiovascular Death
The efficacy of empagliflozin on cardiovascular death was generally consistent across major demographic and disease subgroups.
Vital status was obtained for 99.2% of subjects in the trial. A total of 463 deaths were recorded during the EMPA-REG OUTCOME trial. Most of these deaths were categorized as cardiovascular deaths. The non-cardiovascular deaths were only a small proportion of deaths and were balanced between the treatment groups (2.1% in patients treated with empagliflozin, and 2.4% of patients treated with placebo).
Linagliptin Cardiovascular Safety Trials
CARMELINA
The cardiovascular risk of linagliptin was evaluated in CARMELINA (NCT0189753), a multinational, multi-center, placebo-controlled, double-blind, parallel group trial comparing linagliptin (N=3494) to placebo (N=3485) in adult patients with type 2 diabetes mellitus and a history of established macrovascular and/or renal disease. The trial compared the risk of major adverse cardiovascular events (MACE) between linagliptin and placebo when these were added to standard of care treatments for diabetes and other cardiovascular risk factors. The trial was event driven, the median duration of follow-up was 2.2 years and vital status was obtained for 99.7% of patients.
Patients were eligible to enter the trial if they were adults with type 2 diabetes, with HbA1c of 6.5% to 10%, and had either albuminuria and previous macrovascular disease (39% of enrolled population), or evidence of impaired renal function by eGFR and Urinary Albumin Creatinine Ratio (UACR) criteria (42% of enrolled population), or both (18% of enrolled population).
At baseline the mean age was 66 years and the population was 63% male, 80% Caucasian, 9% Asian, and 6% Black. Mean HbA1c was 8.0% and mean duration of type 2 diabetes mellitus was 15 years. The trial population included 17% patients ≥75 years of age and 62% patients with renal impairment defined as eGFR <60 mL/min/1.73 m2. The mean eGFR was 55 mL/min/1.73 m2 and 27% of patients had mild renal impairment (eGFR 60 to 90 mL/min/1.73 m2), 47% of patients had moderate renal impairment (eGFR 30 to <60 mL/min/1.73 m2) and 15% of patients had severe renal impairment (eGFR <30 mL/min/1.73 m2). Patients were taking at least one antidiabetic drug (97%), and the most common were insulin and analogues (57%), metformin (54%) and sulfonylurea (32%). Patients were also taking antihypertensives (96%), lipid lowering drugs (76%) with 72% on statin, and aspirin (62%).
The primary endpoint, MACE, was the time to first occurrence of one of three composite outcomes which included cardiovascular death, non-fatal myocardial infarction or non-fatal stroke. The study was designed as a non-inferiority trial with a pre-specified risk margin of 1.3 for the hazard ratio of MACE. A total of 434 patients on linagliptin and 420 patients on placebo experienced MACE. The incidence rate of MACE in both treatment arms: 56.3 MACE per 1000 patient-years on placebo vs. 57.7 MACE per 1000 patient-years on linagliptin. The estimated hazard ratio for MACE associated with linagliptin relative to placebo was 1.02 with a 95% confidence interval of (0.89, 1.17). The upper bound of this confidence interval, 1.17, excluded the risk margin of 1.3.
CAROLINA
The cardiovascular risk of linagliptin was evaluated in CAROLINA, a multi-center, multinational, randomized, double-blind parallel group trial comparing linagliptin (N=3023) to glimepiride (N=3010) in adult patients with type 2 diabetes mellitus and a history of established cardiovascular disease and/or multiple cardiovascular risk factors. The trial compared the risk of major adverse cardiovascular events (MACE) between linagliptin and glimepiride when these were added to standard of care treatments for diabetes and other cardiovascular risk factors. The trial was event driven, the median duration of follow-up was 6.23 years and vital status was obtained for 99.3% of patients.
Patients were eligible to enter the trial if they were adults with type 2 diabetes with insufficient glycemic control (defined as HbA1c of 6.5% to 8.5% or 6.5% to 7.5% depending on treatment-naïve, on monotherapy or on combination therapy), and were defined to be at high cardiovascular risk with previous vascular disease, evidence of vascular related end-organ damage, age ≥70 years, and/or two cardiovascular risk factors (duration of diabetes >10 years, systolic blood pressure >140 mmHg, current smoker, LDL cholesterol ≥135 mg/dL).
At baseline the mean age was 64 years and the population was 60% male, 73% Caucasian, 18% Asian, and 5% Black. The mean HbA1c was 7.15% and mean duration of type 2 diabetes was 7.6 years. The trial population included 34% patients ≥70 years of age and 19% patients with renal impairment defined as eGFR <60 mL/min/1.73 m2. The mean eGFR was 77 mL/min/1.73 m2. Patients were taking at least one antidiabetic drug (91%) and the most common were metformin (83%) and sulfonylurea (28%). Patients were also taking antihypertensives (89%), lipid lowering drugs (70%) with 65% on statin, and aspirin (47%).
The primary endpoint, MACE, was the time to first occurrence of one of three composite outcomes which included cardiovascular death, non-fatal myocardial infarction or non-fatal stroke. The study was designed as a non-inferiority trial with a pre-specified risk margin of 1.3 for the hazard ratio of MACE. A total of 356 patients on linagliptin and 362 patients on glimepiride experienced MACE. The incidence rate of MACE in both treatment arms: 20.7 MACE per 1000 patient-years on linagliptin vs. 21.2 MACE per 1000 patient-years on glimepiride. The estimated hazard ratio for MACE associated with linagliptin relative to glimepiride was 0.98 with a 95% confidence interval of (0.84, 1.14). The upper bound of this confidence interval, 1.14, excluded the risk margin of 1.3.
16 How Supplied/storage And Handling
TRIJARDY XR tablets are available as follows:
Tablet Strength Color/Shape Tablet Markings Package Size NDC Number 5 mg Empagliflozin 2.5 mg Linagliptin 1000 mg Metformin HCl Extended-Release grey, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "395" on the top line and "5/2.5" on the bottom line Bottles of 60 Bottles of 180 0597-0395-82 0597-0395-23 10 mg Empagliflozin 5 mg Linagliptin 1000 mg Metformin HCl Extended-Release tan, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "380" on the top line and "10/5" on the bottom line Bottles of 30 Bottles of 90 0597-0380-13 0597-0380-68 12.5 mg Empagliflozin 2.5 mg Linagliptin 1000 mg Metformin HCl Extended-Release red, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "385" on the top line and "12.5/2.5" on the bottom line Bottles of 60 Bottles of 180 0597-0385-77 0597-0385-86 25 mg Empagliflozin 5 mg Linagliptin 1000 mg Metformin HCl Extended-Release brown, oval-shaped, film-coated tablet Printed on one side in white ink with the BI logo and "390" on the top line and "25/5" on the bottom line Bottles of 30 Bottles of 90 0597-0390-71 0597-0390-13 STORAGE AND HANDLING SECTION
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from exposure to high humidity.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Lactic Acidosis
Inform patients of the risks of lactic acidosis due to metformin, its symptoms, and conditions that predispose to its development. Advise patients to discontinue TRIJARDY XR immediately and to notify their healthcare provider promptly if unexplained hyperventilation, malaise, myalgia, unusual somnolence, or other nonspecific symptoms occur. Counsel patients against excessive alcohol intake and inform patients about importance of regular testing of renal function while receiving TRIJARDY XR. Instruct patients to inform their healthcare provider that they are taking TRIJARDY XR prior to any surgical or radiological procedure, as temporary discontinuation may be required until renal function has been confirmed to be normal [see Warnings and Precautions (5.1)].
Pancreatitis
Inform patients that acute pancreatitis has been reported during use of linagliptin. Inform patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue TRIJARDY XR promptly and contact their healthcare provider if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
Ketoacidosis
Inform patients that ketoacidosis is a serious life-threatening condition and that cases of ketoacidosis have been reported during use of empagliflozin, sometimes associated with illness or surgery among other risk factors. Instruct patients to check ketones (when possible) if symptoms consistent with ketoacidosis occur even if blood glucose is not elevated. If symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing) occur, instruct patients to discontinue TRIJARDY XR and seek medical attention immediately [see Warnings and Precautions (5.3)].
Volume Depletion
Inform patients that symptomatic hypotension may occur with TRIJARDY XR and advise them to contact their healthcare provider if they experience such symptoms [see Warnings and Precautions (5.4)]. Inform patients that dehydration may increase the risk for hypotension, and to maintain adequate fluid intake.
Serious Urinary Tract Infections
Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur [see Warnings and Precautions (5.5)].
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Inform patients that the incidence of hypoglycemia is increased when TRIJARDY XR is used in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin, and that a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia [see Warnings and Precautions (5.6)].
Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Inform patients that necrotizing infections of the perineum (Fournier's gangrene) have occurred with empagliflozin, a component of TRIJARDY XR. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4°F or malaise [see Warnings and Precautions (5.7)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.8)].
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
Inform male patients that yeast infection of the penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males and patients with chronic and recurrent infections. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.8)].
Hypersensitivity Reactions
Inform patients that serious allergic reactions, such as anaphylaxis, angioedema, and exfoliative skin conditions, have been reported during postmarketing use of linagliptin or empagliflozin, components of TRIJARDY XR. If symptoms of allergic reactions (such as rash, skin flaking or peeling, urticaria, swelling of the skin, or swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing) occur, patients must stop taking TRIJARDY XR and seek medical advice promptly [see Warnings and Precautions (5.9)].
Vitamin B12 Deficiency
Inform patients about importance of regular hematological parameters while receiving TRIJARDY XR [see Warnings and Precautions (5.10)].
Severe and Disabling Arthralgia
Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs [see Warnings and Precautions (5.11)].
Bullous Pemphigoid
Inform patients that bullous pemphigoid has been reported during use of linagliptin. Instruct patients to seek medical advice if bulers or erosions occur [see Warnings and Precautions (5.12)].
Heart Failure
Inform patients of the signs and symptoms of heart failure. Before initiating TRIJARDY XR, patients should be asked about a history of heart failure or other risk factors for heart failure including moderate to severe renal impairment. Instruct patients to contact their healthcare provider as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight or swelling of the feet [see Warnings and Precautions (5.13)].
Laboratory Tests
Inform patients that elevated glucose in urinalysis is expected when taking TRIJARDY XR.
Pregnancy
Advise pregnant patients, and patients of reproductive potential, of the potential risk to a fetus with treatment with TRIJARDY XR [see Use in Specific Populations (8.1)]. Instruct patients to report pregnancies to their healthcare provider as soon as possible.
Lactation
Advise patients that breastfeeding is not recommended during treatment with TRIJARDY XR [see Use in Specific Populations (8.2)].
Patients of Reproductive Potential
Inform patients that treatment with metformin may result in ovulation in some premenopausal anovulatory patients, which may lead to unintended pregnancy [see Use in Specific Populations (8.3)].
Administration Instructions
Inform patients that the tablets must be swallowed whole and never split, crushed, dissolved, or chewed and that incompletely dissolved TRIJARDY XR tablets may be eliminated in the feces.
Missed Dose
Instruct patients to take TRIJARDY XR only as prescribed. If a dose is missed, it should be taken as soon as the patient remembers. Advise patients not to double their next dose.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA
Marketed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA and Eli Lilly and Company Indianapolis, IN 46285 USA
Licensed from:Boehringer Ingelheim International GmbH, Ingelheim, Germany.
TRIJARDY is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the Jardiance®, Tradjenta®, EMPA-REG OUTCOME®, CARMELINA®, and CAROLINA® trademarks under license.
The other brands uled are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
Copyright © 2022 Boehringer Ingelheim International GmbHALL RIGHTS RESERVED
SPL9015C
COL9014CJ072022
Spl Medguide Section
MEDICATION GUIDE TRIJARDY® XR (try-JAR-dee XR) (empagliflozin, linagliptin, and metformin hydrochloride extended-release tablets) for oral use This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: October 2022    What is the most important information I should know about TRIJARDY XR? TRIJARDY XR can cause serious side effects, including:
1. Lactic Acidosis. Metformin hydrochloride, one of the medicines in TRIJARDY XR, can cause a rare but serious condition called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital. Stop taking TRIJARDY XR and call your healthcare provider right away or go to the nearest hospital emergency room if you get any of the following symptoms of lactic acidosis:
- feel very weak and tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
- have unusual sleepiness or sleep longer than usual
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis with TRIJARDY XR if you:
- have moderate to severe kidney problems.
- have liver problems.
- drink a lot of alcohol (very often or short-term "binge" drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery or other procedure for which you need to restrict the amount of food and liquid you eat and drink.
- have congestive heart failure.
- have a heart attack, severe infection, or stroke.
- are 65 years of age or older.
Tell your healthcare provider if you have any of the problems in the ul above. Tell your healthcare provider that you are taking TRIJARDY XR before you have surgery or x-ray tests. Your healthcare provider may need to stop your TRIJARDY XR for a while if you have surgery or certain x-ray tests. TRIJARDY XR can have other serious side effects. See "What are the possible side effects of TRIJARDY XR?"
2. Inflammation of the pancreas (pancreatitis) which may be severe and lead to death. Certain medical problems make you more likely to get pancreatitis.Before you start taking TRIJARDY XR, tell your healthcare provider if you have ever had:
- inflammation of your pancreas (pancreatitis)
- a history of alcoholism
- stones in your gallbladder (gallstones)
- high blood triglyceride levels
Stop taking TRIJARDY XR and call your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis.
3. Ketoacidosis (increased ketones in your blood or urine). Ketoacidosis has happened in people who have type 1 diabetes or type 2 diabetes, during treatment with empagliflozin, one of the medicines in TRIJARDY XR. Ketoacidosis has also happened in people with diabetes who were sick or who had surgery during treatment with TRIJARDY XR. Ketoacidosis is a serious condition, which needs to be treated in a hospital. Ketoacidosis may lead to death. Ketoacidosis can happen with TRIJARDY XR even if your blood sugar is less than 250 mg/dL. Stop taking TRIJARDY XR and call your healthcare provider right away or go to the nearest hospital emergency room if you get any of the following symptoms:
- nausea
- vomiting
- stomach-area (abdominal) pain
- tiredness
- trouble breathing
If you get any of these symptoms during treatment with TRIJARDY XR, if possible, check for ketones in your urine, even if your blood sugar is less than 250 mg/dL.
4. Dehydration. TRIJARDY XR can cause some people to become dehydrated (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, light-headed, or weak, especially when you stand up (orthostatic hypotension). There have been reports of sudden worsening of kidney function in people who are taking TRIJARDY XR.You may be at higher risk of dehydration if you:
- have kidney problems
- are 65 years of age or older
- are on low sodium (salt) diet
- take medicines to lower your blood pressure, including diuretics (water pills)
Talk to your healthcare provider about what you can do to prevent dehydration including how much fluid you should drink on a daily basis.Talk to your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you are sick or cannot eat, or start to lose liquids from your body, for example from vomiting, diarrhea or being in the sun too long. What is TRIJARDY XR? TRIJARDY XR is a prescription medicine that contains 3 diabetes medicines, empagliflozin (JARDIANCE), linagliptin (TRADJENTA), and metformin hydrochloride. TRIJARDY XR can be used:
- along with diet and exercise to lower blood sugar in adults with type 2 diabetes,
- in adults with type 2 diabetes who have known cardiovascular disease when empagliflozin (JARDIANCE), one of the medicines in TRIJARDY XR, is needed to reduce the risk of cardiovascular death.
- TRIJARDY XR is not for people with type 1 diabetes. It may increase their risk of diabetic ketoacidosis (increased ketones in blood or urine).
- If you have had pancreatitis in the past, it is not known if you have a higher chance of getting pancreatitis while you take TRIJARDY XR.
- It is not known if TRIJARDY XR is safe and effective in children.
Who should not take TRIJARDY XR? Do not take TRIJARDY XR if you:
- have severe kidney problems, end stage renal disease, or are on dialysis.
- have a condition called metabolic acidosis or diabetic ketoacidosis (increased ketones in the blood or urine).
- are allergic to empagliflozin (JARDIANCE), linagliptin (TRADJENTA), metformin, or any of the ingredients in TRIJARDY XR. See the end of this Medication Guide for a complete ul of ingredients in TRIJARDY XR. Symptoms of a serious allergic reaction to TRIJARDY XR may include:
- skin rash, itching, flaking or peeling
- raised red patches on your skin (hives)
- swelling of your face, lips, tongue and throat that may cause difficulty in breathing or swallowing
- difficulty with swallowing or breathing
If you have any of these symptoms, stop taking TRIJARDY XR and call your healthcare provider right away or go to the nearest hospital emergency room. What should I tell my healthcare provider before taking TRIJARDY XR? Before taking TRIJARDY XR, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are 65 years of age or older.
- have a history of infection of the vagina or penis.
- have a history of urinary tract infection or problems with urination.
- are going to have surgery. Your healthcare provider may stop your TRIJARDY XR before you have surgery. Talk to your healthcare provider if you are having surgery about when to stop taking TRIJARDY XR and when to start it again.
- are eating less, or there is a change in your diet.
- have or have had problems with your pancreas, including pancreatitis or surgery on your pancreas.
- drink alcohol very often or drink a lot of alcohol in the short term ("binge" drinking).
- are going to get an injection of dye or contrast agents for an x-ray procedure. TRIJARDY XR may need to be stopped for a short time. Talk to your healthcare provider about when you should stop TRIJARDY XR and when you should start TRIJARDY XR again. See "What is the most important information I should know about TRIJARDY XR?"
- have type 1 diabetes. TRIJARDY XR should not be used to treat people with type 1 diabetes.
- have low levels of vitamin B12 in your blood.
- are pregnant or plan to become pregnant. TRIJARDY XR may harm your unborn baby. If you become pregnant while taking TRIJARDY XR, tell your healthcare provider as soon as possible. Talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are breastfeeding or plan to breastfeed. TRIJARDY XR may pass into your breast milk and may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you are taking TRIJARDY XR. Do not breastfeed while taking TRIJARDY XR.
- are a person who has not gone through menopause (premenopausal) who does not have periods regularly or at all. TRIJARDY XR can cause the release of an egg from an ovary in a person (ovulation). This can increase your chance of getting pregnant. Tell your healthcare provider right away if you become pregnant while taking TRIJARDY XR.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TRIJARDY XR may affect the way other medicines work, and other medicines may affect how TRIJARDY XR works. Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take TRIJARDY XR?
- Take TRIJARDY XR exactly as your healthcare provider tells you to take it.
- Take TRIJARDY XR by mouth 1 time each day with a meal in the morning. Taking TRIJARDY XR with a meal may lower your chance of having an upset stomach.
- Swallow TRIJARDY XR tablets whole. Do not break, cut, crush, dissolve, or chew TRIJARDY XR. If you cannot swallow TRIJARDY XR tablets whole, tell your healthcare provider.
- You may see something that looks like the TRIJARDY XR tablet in your stool (bowel movement). This is not harmful and should not affect the way TRIJARDY XR works to control your diabetes.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take two doses of TRIJARDY XR at the same time.
- Your healthcare provider may tell you to take TRIJARDY XR along with other diabetes medicines. Low blood sugar can happen more often when TRIJARDY XR is taken with certain other diabetes medicines. See "What are the possible side effects of TRIJARDY XR?"
- If you take too much TRIJARDY XR, call your healthcare provider or local poison control center or go to the nearest hospital emergency room right away.
- Your healthcare provider will do blood tests to check how well your kidneys are working before and during your treatment with TRIJARDY XR.
- When taking TRIJARDY XR, you may have sugar in your urine, which will show up on a urine test.
What should I avoid while taking TRIJARDY XR? Avoid drinking alcohol very often or drinking a lot of alcohol in a short period of time ("binge" drinking). It can increase your chances of getting serious side effects. What are the possible side effects of TRIJARDY XR? TRIJARDY XR may cause serious side effects, including:
- Serious urinary tract infections. Serious urinary tract infections that may lead to hospitalization have happened in people who are taking empagliflozin, one of the medicines in TRIJARDY XR. Tell your healthcare provider if you have any signs or symptoms of a urinary tract infection such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people also may have a fever, back pain, nausea or vomiting.
- Low blood sugar (hypoglycemia). If you take TRIJARDY XR with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take TRIJARDY XR. Signs and symptoms of low blood sugar may include:
- headache
- drowsiness
- weakness
- irritability
- hunger
- fast heartbeat
- confusion
- shaking or feeling jittery
- dizziness
- sweating
- A rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum). Necrotizing fasciitis of the perineum has happened in women and men who take empagliflozin, one of the medicines in TRIJARDY XR. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries, and may lead to death. Seek medical attention immediately if you have a fever or you are feeling very weak, tired or uncomfortable (malaise), and you develop any of the following symptoms in the area between and around your anus and genitals:
- pain or tenderness
- swelling
- redness of skin (erythema)
- Vaginal yeast infection. Symptoms of a vaginal yeast infection include:
- vaginal odor
- vaginal itching
- white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese)
- Yeast infection of the penis (balanitis or balanoposthitis). Swelling of an uncircumcised penis may develop that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of a yeast infection of the penis include:
- redness, itching, or swelling of the penis
- foul smelling discharge from the penis
- rash of the penis
- pain in the skin around the penis
Talk to your healthcare provider about what to do if you get symptoms of a yeast infection of the vagina or penis. Your healthcare provider may suggest you use an over-the-counter antifungal medicine. Talk to your healthcare provider right away if you use an over-the-counter antifungal medication and your symptoms do not go away.
- Allergic (hypersensitivity) reactions. Serious allergic reactions have happened in people who are taking TRIJARDY XR. Symptoms may include:
If you have any of these symptoms, stop taking TRIJARDY XR and call your healthcare provider right away or go to the nearest hospital emergency room.
- swelling of your face, lips, tongue, throat, and other areas on your skin
- difficulty with swallowing or breathing
- raised, red areas on your skin (hives)
- skin rash, itching, flaking, or peeling
- Low vitamin B12 (vitamin B12 deficiency). Using metformin for long periods of time may cause a decrease in the amount of vitamin B12 in your blood, especially if you have had low vitamin B12 blood levels before. Your healthcare provider may do blood tests to check your vitamin B12 levels.
- Joint pain. Some people who take medicines called DPP-4 inhibitors, one of the medicines in TRIJARDY XR, may develop joint pain that can be severe. Call your healthcare provider if you have severe joint pain.
- Skin reaction. Some people who take medicines called DPP-4 inhibitors, one of the medicines in TRIJARDY XR, may develop a skin reaction called bullous pemphigoid that can require treatment in a hospital. Tell your healthcare provider right away if you develop bulers or the breakdown of the outer layer of your skin (erosion). Your healthcare provider may tell you to stop taking TRIJARDY XR.
- Heart failure. Heart failure means your heart does not pump blood well enough. Before you start taking TRIJARDY XR, tell your healthcare provider if you have ever had heart failure or have problems with your kidneys. Contact your healthcare provider right away if you have any of the following symptoms:
These may be symptoms of heart failure.
- increasing shortness of breath or trouble breathing, especially when you lie down
- swelling or fluid retention, especially in the feet, ankles or legs
- an unusually fast increase in weight
- unusual tiredness
The most common side effects of TRIJARDY XR include:
- upper respiratory tract infection
- urinary tract infection
- stuffy or runny nose and sore throat
- diarrhea
- constipation
- headache
- inflammation of the stomach and intestine (gastroenteritis)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of TRIJARDY XR. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store TRIJARDY XR?
- Store TRIJARDY XR at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TRIJARDY XR tablets dry.
- Keep TRIJARDY XR and all medicines out of the reach of children.
General information about the safe and effective use of TRIJARDY XR. Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use TRIJARDY XR for a condition for which it was not prescribed. Do not give TRIJARDY XR to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TRIJARDY XR that is written for health professionals. What are the ingredients in TRIJARDY XR? Active ingredients: empagliflozin, linagliptin, and metformin hydrochloride Inactive ingredients: Tablet core contains: polyethylene oxide, hypromellose, and magnesium stearate. The Film Coatings and Printing Ink contain: hydroxypropyl cellulose, hypromellose, talc, titanium dioxide, arginine, polyethylene glycol, carnauba wax, purified water, shellac glaze, n-butyl alcohol, propylene glycol, ammonium hydroxide, isopropyl alcohol, ferrosoferric oxide and ferric oxide yellow (5 mg/2.5 mg/1000 mg and 25 mg/5 mg/1000 mg), ferric oxide yellow and ferric oxide red (10 mg/5 mg/1000 mg), and ferrosoferric oxide and ferric oxide red (12.5 mg/2.5 mg /1000 mg). Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA Marketed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA and Eli Lilly and Company, Indianapolis, IN 46285 USA Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany TRIJARDY is a registered trademark of and used under license from Boehringer Ingelheim International GmbH. Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the Jardiance®, Tradjenta®, EMPA-REG OUTCOME®, CARMELINA®, and CAROLINA® trademarks under license. The other brands uled are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc. Copyright © 2022 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED COL9014CIJ072022 For more information about TRIJARDY XR, including current prescribing information and Medication Guide, go to www.trijardyxr.com, scan the code, or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

Principal Display Panel - 5 Mg/2.5 Mg/1000 Mg Tablet Bottle Label
NDC 0597-0395-82
Trijardy®XR(empagliflozin, linagliptin, and metformin hydrochloride extended-release tablets)
5 mg/2.5 mg/1000 mg
DISPENSE WITH ACCOMPANYING MEDICATION GUIDE
60 tabletsRx only
BoehringerIngelheim
Lilly
Principal Display Panel - 10 Mg/5 Mg/1000 Mg Tablet Bottle Label
NDC 0597-0380-13
Trijardy®XR(empagliflozin, linagliptin, and metformin hydrochloride extended-release tablets)
10 mg/5 mg/1000 mg
DISPENSE WITH ACCOMPANYINGMEDICATION GUIDE
30 tabletsRx only
BoehringerIngelheim
Lilly
Principal Display Panel - 12.5 Mg/2.5 Mg/1000 Mg Tablet Bottle Label
NDC 0597-0385-86
Trijardy®XR(empagliflozin, linagliptin, and metformin hydrochloride extended-release tablets)
12.5 mg/2.5 mg/1000 mg
DISPENSE WITH ACCOMPANYINGMEDICATION GUIDE
180 tabletsRx only
BoehringerIngelheim
Lilly

Principal Display Panel - 25 Mg/5 Mg/1000 Mg Tablet Bottle Label
NDC 0597-0390-13
Trijardy®XR(empagliflozin, linagliptin, and metformin hydrochloride extended-release tablets)
25 mg/5 mg/1000 mg
DISPENSE WITH ACCOMPANYINGMEDICATION GUIDE
90 tabletsRx only
BoehringerIngelheim
Lilly

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


