Miglitol Dailymed
Generic: miglitol is used for the treatment of Colitis, Ulcerative Diabetes Mellitus, Type 2 Digestive System Abnormalities Intestinal Pseudo-Obstruction Malabsorption Syndromes Diabetic Ketoacidosis
Go PRO for all pill images
For Oral Use
Description Section
Miglitol Tablets, an oral alpha-glucosidase inhibitor for use in the management of non-insulin-dependent diabetes mellitus (NIDDM). Miglitol is a desoxynojirimycin derivative, and is chemically known as 3,4,5-piperidinetriol, 1-(2-hydroxyethyl) -2-(hydroxymethyl)-, [2R-(2a,3ß,4a, 5ß)]-. It is a white to pale-yellow powder with a molecular weight of 207.2. Miglitol is soluble in water and has a pK a of 5.9. Its empirical formula is C 8 H 17 NO 5 and its chemical structure is as follows: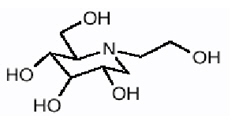
Miglitol tablets are available as 25 mg, 50 mg, and 100 mg tablets for oral use. The inactive ingredients are corn starch, microcrystalline cellulose, magnesium stearate, hypromelloses, polyethylene glycols, titanium dioxide, and polysorbate 80.
Clinical Pharmacology Section
Miglitol is a desoxynojirimycin derivative that delays the digestion of ingested carbohydrates, thereby resulting in a smaller rise in blood glucose concentration following meals. As a consequence of plasma glucose reduction, miglitol tablets reduce levels of glycosylated hemoglobin in patients with Type II (non-insulin-dependent) diabetes mellitus. Systemic nonenzymatic protein glycosylation, as reflected by levels of glycosylated hemoglobin, is a function of average blood glucose concentration over time.
Mechanism of Action
In contrast to sulfonylureas, miglitol tablets do not enhance insulin secretion. The antihyperglycemic action of miglitol results from a reversible inhibition of membrane-bound intestinal α-glucoside hydrolase enzymes. Membrane-bound intestinal α-glucosidases hydrolyze oligosaccharides and disaccharides to glucose and other monosaccharides in the brush border of the small intestine. In diabetic patients, this enzyme inhibition results in delayed glucose absorption and lowering of postprandial hyperglycemia.
Because its mechanism of action is different, the effect of miglitol tablets to enhance glycemic control is additive to that of sulfonylureas when used in combination. In addition, miglitol tablets diminish the insulinotropic and weight-increasing effects of sulfonylureas.
Miglitol has minor inhibitory activity against lactase and consequently, at the recommended doses, would not be expected to induce lactose intolerance.
PHARMACOKINETICS SECTION
Absorption of miglitol is saturable at high doses: a dose of 25 mg is completely absorbed, whereas a dose of 100 mg is 50% to 70% absorbed. For all doses, peak concentrations are reached in 2 to 3 hours. There is no evidence that systemic absorption of miglitol contributes to its therapeutic effect.
The protein binding of miglitol is negligible (<4.0%). Miglitol has a volume of distribution of 0.18 L/kg, consistent with distribution primarily into the extracellular fluid.
Miglitol is not metabolized in humans or in any animal species studied. No metabolites have been detected in plasma, urine, or feces, indicating a lack of either systemic or pre-systemic metabolism.
Miglitol is eliminated by renal excretion as unchanged drug. Following a 25 mg dose, over 95% of the dose is recovered in the urine within 24 hours. At higher doses, the cumulative recovery of drug from urine is somewhat lower due to the incomplete bioavailability. The elimination half-life of miglitol from plasma is approximately 2 hours.
Because miglitol is excreted primarily by the kidneys, accumulation of miglitol is expected in patients with renal impairment. Patients with creatinine clearance <25 mL/min taking 25 mg 3 times daily, exhibited a greater than two-fold increase in miglitol plasma levels as compared to subjects with creatinine clearance >60 mL/min. Dosage adjustment to correct the increased plasma concentrations is not feasible because miglitol acts locally. Little information is available on the safety of miglitol in patients with creatinine clearance <25 mL/min. Therefore, treatment of these patients with miglitol is not recommended.
Miglitol pharmacokinetics were not altered in cirrhotic patients relative to healthy control subjects. Since miglitol is not metabolized, no influence of hepatic function on the kinetics of miglitol is expected.
No significant difference in the pharmacokinetics of miglitol was observed between elderly men and women when body weight was considered.
Several pharmacokinetic studies were conducted in Japanese volunteers, with results similar to those observed in Caucasians. A study comparing the pharmacodynamic response to a single 50 mg dose in Black and Caucasian healthy volunteers indicated similar glucose and insulin responses in both populations.
Clinical Studies Section
Miglitol tablets were evaluated in two U.S. and three non-U.S. controlled, fixed-dose, monotherapy studies, in which 735 patients treated with miglitol tablets were evaluated for efficacy analyses (see Table 1 ).
In Study 1, a 1-year study in which miglitol tablets were evaluated as monotherapy and also as combination therapy, there was a statistically significantly smaller increase in mean glycosylated hemoglobin (HbA1c) over time in the miglitol 50 mg 3 times daily monotherapy arm compared to placebo. Significant reductions in mean fasting and postprandial plasma glucose levels and in mean postprandial insulin levels were observed in patients treated with miglitol tablets compared with the placebo group.
In Study 2, a 14-week study, there was a significant decrease in HbA1c in patients receiving miglitol tablets 50 mg 3 times daily or 100 mg 3 times daily compared to placebo. In addition, there were significant reductions in postprandial plasma glucose and postprandial serum insulin levels compared to placebo.
Study 3 was a 6-month dose-ranging trial evaluating miglitol tablets at doses from 25 mg 3 times daily, to 200 mg 3 times daily. Miglitol tablets produced a greater reduction in HbA1c than placebo at all doses, although the effect was statistically significant at the 100 mg 3 times daily and 200 mg 3 times daily. In addition, all doses of miglitol tablets produced significant reductions in postprandial plasma glucose and postprandial insulin levels compared to placebo.
Studies 4 and 5 were 6-month studies evaluating miglitol tablets at 50 and 100 mg 3 times daily, and 100 mg 3 times daily, respectively. As compared to placebo, miglitol tablets produced reductions in HbA1c, as well as a significant reduction in postprandial plasma glucose in both studies at the doses employed.
Table 1 Results of Monotherapy Study with Miglitol Tablets
HbA1c (%) 1-hour Postprandial Glucose (mg/dL) Study Treatment Mean Change from Baseline Mean baseline ranged from 7.54 to 8.72% in these studies. Treatment Effect The result of subtracting the placebo group average. Mean Change from Baseline Treatment Effect 2 1 Placebo +0.71 --- +24 --- (U.S.) Miglitol tablets 50 mg 3 times daily +0.13 -0.58 p≤ 0.05 -39 -63 3 2 Placebo +0.47 --- +15 --- (U.S.) Miglitol tablets 50 mg 3 times daily -0.22 -0.69 3 -52 -67 3 Miglitol tablets 100 mg 3 times daily -0.28 -0.75 3 -59 -74 3 3 Placebo +0.18 --- +2 --- (non-U.S.) Miglitol tablets 25 mg 3 times daily -0.08 -0.26 -33 -35 3 Miglitol tablets 50 mg 3 times daily -0.22 -0.40 -45 -47 3 Miglitol tablets 100 mg 3 times daily -0.63 -0.81 3 -62 -64 3 Miglitol tablets 200 mg 3 times daily Although results for the 200 mg 3 times daily are presented for completeness, the maximum recommended dosage of Miglitol tablets is 100 mg 3 times daily. -0.84 -1.02 3 -85 -87 3 4 Placebo +0.01 --- +8 --- (non-U.S.) Miglitol tablets 50 mg 3 times daily -0.35 -0.36 3 -20 -28 3 Miglitol tablets 100 mg 3 times daily -0.57 -0.58 3 -25 -33 3 5 Placebo +0.32 --- +17 --- (non-U.S.) Miglitol tablets 100 mg 3 times daily -0.43 -0.75 3 -38 -55 3
Miglitol tablets were studied as adjunctive therapy to a background of maximal or near-maximal sulfonylurea (SFU) treatment in three large, double-blind, randomized studies (two U.S. and one non-U.S.) in which 471 patients treated with miglitol tablets were evaluated for efficacy (see Table 2 ).
Study 6 included patients under treatment with maximal doses of SFU at entry. At the end of this 14-week study, the mean treatment effects on glycosylated hemoglobin (HbA1c) were -0.82% and -0.74% for patients receiving miglitol tablets 50 mg 3 times daily plus SFU, and miglitol tablets 100 mg 3 times daily plus SFU, respectively.
Study 7 was a 1-year study in which miglitol tablets at 25, 50 or 100 mg 3 times daily was added to a maximal dose of glyburide (10 mg twice daily). At the end of this study, the mean treatment effects on HbA1c of miglitol tablets when added to maximum glyburide therapy were -0.30%, -0.62%, and -0.73% with the 25, 50 and 100 mg 3 times daily dosages of miglitol tablets, respectively.
In Study 8, the addition of miglitol tablets 100 mg 3 times daily to a background of treatment with glyburide produced an additional mean treatment effect on HbA1c of -0.66%.
Table 2 Results of Combination Therapy with Miglitol Tablets Plus Sulfonylurea (SFU) HbA1c (%) 1-hour Postprandial Glucose (mg/dL) Study Treatment Mean Change from Baseline Mean baseline ranged from 8.56 to 9.16% in these studies. Treatment Effect The result of subtracting the placebo group average. Mean Change from Baseline Treatment Effect 2 6 Placebo + SFU +0.33 --- -1 --- (U.S.) Miglitol tablets 50 mg 3 times daily + SFU -0.49 -0.82 p≤ 0.05 -69 -68 3 Miglitol tablets 100 mg 3 times daily + SFU -0.41 -0.74 3 -73 -72 3 7 Placebo + SFU +1.01 --- 48 --- (U.S.) Miglitol tablets 25 mg 3 times daily + SFU +0.71 -0.30 -2 -50 3 Miglitol tablets 50 mg 3 times daily + SFU +0.39 -0.62 3 -13 -61 3 Miglitol tablets 100 mg 3 times daily + SFU +0.28 -0.73 3 -33 -81 3 8 Placebo + SFU +0.16 --- +10 --- (non-U.S.) Miglitol tablets 100 mg 3 times daily + SFU -0.50 -0.66 3 -36 -46 3
Results from controlled, fixed-dose studies of miglitol tablets as monotherapy or as combination treatment with a sulfonylurea were combined to derive a pooled estimate of the difference from placebo in the mean change from baseline in glycosylated hemoglobin (HbA1c) and postprandial plasma glucose as shown in Figures 1 and 2: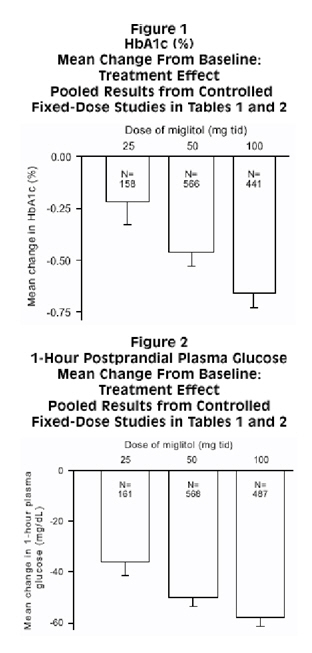
Because of its mechanism of action, the primary pharmacologic effect of miglitol is manifested as a reduction in postprandial plasma glucose, as shown previously in all of the major clinical trials. Miglitol tablets were statistically significantly different from placebo at all doses in each of the individual studies with respect to effect on mean one-hour postprandial plasma glucose, and there is a dose response from 25 to 100 mg 3 times daily for this efficacy parameter.
Indications & Usage Section
Miglitol tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Contraindications Section
Miglitol tablets are contraindicated in patients with:
- Diabetic ketoacidosis
- Inflammatory bowel disease, colonic ulceration, or partial intestinal obstruction, and in patients predisposed to intestinal obstruction
- Chronic intestinal diseases associated with marked disorders of digestion or absorption, or with conditions that may deteriorate as a result of increased gas formation in the intestine
- Hypersensitivity to the drug or any of its components
Precautions Section
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with miglitol tablets or any other anti-diabetic drug.
GENERAL PRECAUTIONS SECTION
Because of its mechanism of action, miglitol tablets, when administered alone should not cause hypoglycemia in the fasted or postprandial state. Sulfonylureas and insulin can cause hypoglycemia. Because miglitol tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood glucose, it may increase the hypoglycemic potential of the sulfonylurea or insulin. Consider reducing the dose of sulfonylureas or insulin when miglitol tablets are used in combination with these medications.
Oral glucose (dextrose), whose absorption is not delayed by miglitol tablets, should be used instead of sucrose (cane sugar) in the treatment of mild-to-moderate hypoglycemia. Sucrose, whose hydrolysis to glucose and fructose is inhibited by miglitol tablets, is unsuitable for the rapid correction of hypoglycemia. Severe hypoglycemia may require the use of either intravenous glucose infusion or glucagon injection.
When diabetic patients are exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times, temporary insulin therapy may be necessary.
Plasma concentrations of miglitol tablets in renally impaired volunteers were proportionally increased relative to the degree of renal dysfunction. Long-term clinical trials in diabetic patients with significant renal dysfunction (serum creatinine >2.0 mg/dL) have not been conducted. Therefore, treatment of these patients with miglitol tablets is not recommended.
Information for Patients
The following information should be provided to patients:
- Miglitol tablets should be taken orally three times a day at the start of each main meal. It is important to continue to adhere to dietary instructions, a regular exercise program, and regular testing of urine and/or blood glucose.
- Miglitol tablets themselves do not cause hypoglycemia when administered to patients in the fasted state. Sulfonylurea drugs and insulin, however, can lower blood sugar levels and cause symptoms or life-threatening hypoglycemia. Because miglitol tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood sugar, it may increase the hypoglycemic potential of these agents. The risk of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be well understood by patients and responsible family members. Because miglitol tablets prevent the breakdown of table sugar, a source of glucose (dextrose, D-glucose) should be readily available to treat symptoms of low blood sugar when taking miglitol tablets in combination with a sulfonylurea or insulin.
- If side effects occur with miglitol tablets, they usually develop during the first few weeks of therapy. They are most commonly mild-to-moderate dose-related gastrointestinal effects, such as flatulence, soft stools, diarrhea, or abdominal discomfort, and generally diminish in frequency and intensity with time. Discontinuation of drug usually results in rapid resolution of these gastrointestinal symptoms.
In initiating treatment for type 2 diabetes, diet should be emphasized as the primary form of treatment. Caloric restriction and weight loss are essential in the obese diabetic patient. Proper dietary management alone may be effective in controlling the blood glucose and symptoms of hyperglycemia. The importance of regular physical activity should also be stressed, and cardiovascular risk factors should be identified and corrective measures taken where possible. Use of miglitol tablets or other antidiabetic medications must be viewed by both the physician and patient as a treatment in addition to diet and not as a substitution or as a convenient mechanism for avoiding dietary restraint. Furthermore, loss of blood glucose control on diet alone may be transient, thus requiring only short-term administration of miglitol tablets or other antidiabetic medications. Maintenance or discontinuation of miglitol tablets or other antidiabetic medications should be based on clinical judgment using regular clinical and laboratory evaluations.
LABORATORY TESTS SECTION
Therapeutic response to miglitol tablets may be monitored by periodic blood glucose tests. Measurement of glycosylated hemoglobin levels is recommended for the monitoring of long-term glycemic control.
DRUG INTERACTIONS SECTION
Several studies investigated the possible interaction between miglitol and glyburide. In six healthy volunteers given a single dose of 5 mg glyburide on a background of 6 days treatment with miglitol (50 mg 3 times daily for 4 days followed by 100 mg 3 times daily for 2 days) or placebo, the mean C max and AUC values for glyburide were 17% and 25% lower, respectively, when glyburide was given with miglitol. In a study in diabetic patients in which the effects of adding miglitol 100 mg 3 times daily for 7 days or placebo to a background regimen of 3.5 mg glyburide daily were investigated, the mean AUC value for glyburide was 18% lower in the group treated with miglitol, although this difference was not statistically significant. Information on a potential interaction with glyburide was obtained from one of the large U.S. clinical trials (Study 7) in which patients were dosed with either miglitol or placebo on a background of glyburide 10 mg twice daily. At the 6-month and 1-year clinic visits, patients taking concomitant miglitol 100 mg 3 times daily exhibited mean C max values for glyburide that were 16% and 8% lower, respectively, compared to patients taking glyburide alone. However, these differences were not statistically significant. Thus, although there was a trend toward lower AUC and C max values for glyburide when co-administered with miglitol tablets, no definitive statement regarding a potential interaction can be made based on the foregoing three studies.
The effect of miglitol (100 mg 3 times daily for 7 days) on the pharmacokinetics of a single 1000 mg dose of metformin was investigated in healthy volunteers. Mean AUC and C max values for metformin were 12% to 13% lower when the volunteers were given miglitol as compared with placebo, but this difference was not statistically significant.
In a study with healthy volunteers, co-administration of either 50 mg or 100 mg miglitol 3 times daily together with digoxin reduced the average plasma concentrations of digoxin by 19% and 28%, respectively. However, in diabetic patients under treatment with digoxin, plasma digoxin concentrations were not altered by co-administration of miglitol 100 mg 3 times daily for 14 days.
Other healthy volunteer studies have demonstrated that miglitol may significantly reduce the bioavailability of ranitidine and propranolol by 60% and 40%, respectively. No effect of miglitol was observed on the pharmacokinetics or pharmacodynamics of either warfarin or nifedipine.
Intestinal adsorbents (e.g., charcoal) and digestive enzyme preparations containing carbohydrate-splitting enzymes (e.g., amylase, pancreatin) may reduce the effect of miglitol tablets and should not be taken concomitantly.
In 12 healthy males, concomitantly administered antacid did not influence the pharmacokinetics of miglitol.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Miglitol was administered to mice by the dietary route at doses as high as approximately 500 mg/kg body weight (corresponding to greater than 5 times the exposure in humans based on AUC) for 21 months. In a two-year rat study, miglitol was administered in the diet at exposures comparable to the maximum human exposures based on AUC. There was no evidence of carcinogenicity resulting from dietary treatment with miglitol.
In vitro, miglitol was found to be nonmutagenic in the bacterial mutagenesis (Ames) assay and the eukaryotic forward mutation assay (CHO/HGPRT). Miglitol did not have any clastogenic effects in vivo in the mouse micronucleus test. There were no heritable mutations detected in dominant lethal assay.
A combined male and female fertility study conducted in Wistar rats treated orally with miglitol at dose levels of 300 mg/kg body weight (approximately 8 times the maximum human exposure based on body surface area) produced no untoward effect on reproductive performance or capability to reproduce. Survival, growth, development, and fertility of the offspring were not compromised.
PREGNANCY SECTION
The safety of miglitol tablets in pregnant women has not been established. Developmental toxicology studies have been performed in rats at doses of 50, 150 and 450 mg/kg, corresponding to levels of approximately 1.5, 4, and 12 times the maximum recommended human exposure based on body surface area. In rabbits, doses of 10, 45, and 200 mg/kg corresponding to levels of approximately 0.5, 3, and 10 times the human exposure were examined. These studies revealed no evidence of fetal malformations attributable to miglitol. Doses of miglitol up to 4 and 3 times the human dose (based on body surface area), for rats and rabbits respectively, did not reveal evidence of impaired fertility or harm to the fetus. The highest doses tested in these studies, 450 mg/kg in the rat and 200 mg/kg in the rabbit promoted maternal and/or fetal toxicity. Fetotoxicity was indicated by a slight but significant reduction in fetal weight in the rat study and slight reduction in fetal weight, delayed ossification of the fetal skeleton and increase in the percentage of non-viable fetuses in the rabbit study. In the peri-postnatal study in rats, the NOAEL (No Observed Adverse Effect Level) was 100 mg/kg (corresponding to approximately four times the exposure to humans, based on body surface area). An increase in stillborn progeny was noted at the high dose (300 mg/kg) in the rat peri-postnatal study, but not at the high dose (450 mg/kg) in the delivery segment of the rat developmental toxicity study. Otherwise, there was no adverse effect on survival, growth, development, behavior, or fertility in either the rat developmental toxicity or peri-postnatal studies. There are however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, miglitol should be used during pregnancy only if clearly needed.
NURSING MOTHERS SECTION
Miglitol has been shown to be excreted in human milk to a very small degree. Total excretion into milk accounted for 0.02% of a 100 mg maternal dose. The estimated exposure to a nursing infant is approximately 0.4% of the maternal dose. Although the levels of miglitol reached in human milk are exceedingly low, it is recommended that miglitol tablets not be administered to a nursing woman.
PEDIATRIC USE SECTION
Safety and effectiveness of miglitol tablets in pediatric patients have not been established.
GERIATRIC USE SECTION
Of the total number of subjects in clinical studies of miglitol tablets in the United States, patients valid for safety analyses included 24% over 65, and 3% over 75. No overall differences in safety and effectiveness were observed between these subjects and younger subjects. The pharmacokinetics of miglitol were studied in elderly and young males (n=8 per group). At the dosage of 100 mg 3 times daily for 3 days, no differences between the two groups were found.
Adverse Reactions Section
Gastrointestinal symptoms are the most common reactions to miglitol tablets. In U.S. placebo-controlled trials, the incidences of abdominal pain, diarrhea, and flatulence were 11.7%, 28.7%, and 41.5% respectively in 962 patients treated with miglitol tablets, 25 mg to 100 mg 3 times daily, whereas the corresponding incidences were 4.7%, 10.0%, and 12.0% in 603 placebo-treated patients. The incidence of diarrhea and abdominal pain tended to diminish with continued treatment.
Skin rash was reported in 4.3% of patients treated with miglitol tablets compared to 2.4% of placebo-treated patients. Rashes were generally transient and most were assessed as unrelated to miglitol tablets by physician investigators.
Low serum iron occurred more often in patients treated with miglitol tablets (9.2%) than in placebo-treated patients (4.2%) but did not persist in the majority of cases and was not associated with reductions in hemoglobin or changes in other hematologic indices.
Postmarketing Experience
The following adverse reactions have been reported during post-approval use of miglitol tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: ileus (including paralytic ileus), subileus, gastrointestinal pain, nausea, abdominal distention.
- Pneumatosis Cystoides Intestinalis
There have been rare postmarketing reports of pneumatosis cystoides intestinalis associated with the use of alpha-glucosidase inhibitors, including miglitol tablets. Pneumatosis cystoides intestinalis may present with symptoms of diarrhea, mucus discharge, rectal bleeding, and constipation. Complications may include pneumoperitoneum, volvulus, intestinal obstruction, intussusception, intestinal hemorrhage, and intestinal perforation. If pneumatosis cystoides intestinalis is suspected, discontinue miglitol tablets and perform the appropriate diagnostic imaging.
Overdosage Section
Unlike sulfonylureas or insulin, an overdose of miglitol tablets will not result in hypoglycemia. An overdose may result in transient increases in flatulence, diarrhea, and abdominal discomfort. Because of the lack of extra-intestinal effects seen with miglitol tablets, no serious systemic reactions are expected in the event of an overdose.
Dosage & Administration Section
There is no fixed dosage regimen for the management of diabetes mellitus with miglitol tablets or any other pharmacologic agent. Dosage of miglitol tablets must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended dosage of 100 mg 3 times daily. Miglitol tablets should be taken three times daily at the start of each main meal. Miglitol tablets should be started at 25 mg, and the dosage gradually increased both to reduce gastrointestinal adverse effects and to permit identification of the minimum dose required for adequate glycemic control of the patient.
During treatment initiation and dose titration, one-hour postprandial plasma glucose may be used to determine the therapeutic response to miglitol tablets and identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately 3 months. The therapeutic goal should be to decrease both postprandial plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of miglitol tablets, either as monotherapy or in combination with a sulfonylurea.
The recommended starting dosage of miglitol tablets is 25 mg, given orally three times daily at the start of each main meal. However, some patients may benefit by starting at 25 mg once daily to minimize gastrointestinal adverse effects, and gradually increasing the frequency of administration to 3 times daily.
The usual maintenance dose of miglitol tablets is 50 mg taken 3 times daily, although some patients may benefit from increasing the dose to 100 mg 3 times daily. To allow adaptation to potential gastrointestinal adverse effects, it is recommended that miglitol tablets therapy be initiated at a dosage of 25 mg 3 times daily, then gradually titrated upward to allow adaptation. After 4 to 8 weeks of the 25 mg 3 times daily regimen, the dosage should be increased to 50 mg 3 times daily for approximately three months, following which a glycosylated hemoglobin level should be measured to assess therapeutic response. If at that time, the glycosylated hemoglobin level is not satisfactory, the dosage may be further increased to 100 mg 3 times daily, the maximum recommended dosage. Pooled data from controlled studies suggest a dose-response for both HbA1c and one-hour postprandial plasma glucose throughout the recommended dosage range. However, no single study has examined the effect on glycemic control of titrating patients' doses upwards within the same study. If no further reduction in postprandial glucose or glycosylated hemoglobin levels is observed with titration to 100 mg 3 times daily, consideration should be given to lowering the dose. Once an effective and tolerated dosage is established, it should be maintained.
The maximum recommended dosage of miglitol tablets is 100 mg 3 times daily. In one clinical trial, 200 mg 3 times daily gave additional improved glycemic control but increased the incidence of the gastrointestinal symptoms described above.
Patients Receiving Sulfonylureas
Sulfonylurea agents may cause hypoglycemia. There was no increased incidence of hypoglycemia in patients who took miglitol tablets in combination with sulfonylurea agents compared to the incidence of hypoglycemia in patients receiving sulfonylureas alone in any clinical trial. However, miglitol tablets given in combination with a sulfonylurea will cause a further lowering of blood glucose and may increase the risk of hypoglycemia due to the additive effects of the two agents. If hypoglycemia occurs, appropriate adjustments in the dosage of these agents should be made (see PRECAUTIONS ).
How Supplied Section
Miglitol tablets are available as 25 mg, 50 mg, and 100 mg white to off-white, circular, biconvex film-coated tablets, debossed with the logo-mark "OP" on one side and the product code on the other side, as indicated below.
Tablet Identification Strength NDC Front Back Bottles of 100: 25 mg 69367-303-01 OP 25 50 mg 69367-304-01 OP 26 100 mg 69367-305-01 OP 27 STORAGE AND HANDLING SECTION
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Manufactured by: Orient Pharma Co., Ltd. Yunlin, Taiwan
Distributed by: Westminster Pharmaceuticals, LLC
Nashville, TN 37217
Rev. 10/2020
Recent Major Changes Section
Package Label.principal Display Panel
NDC 69367-303-01
Miglitol Tablets 25 mg
Rx only
100 Tablets

Package Label.principal Display Panel
NDC 69367-304-01
Miglitol Tablets 50 mg
Rx only
100 Tablets

Package Label.principal Display Panel
NDC 69367-305-01
Miglitol Tablets 100 mg
Rx only
100 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


