nystatin (nystatin 500000 [usp'u]) Dailymed
Generic: nystatin is used for the treatment of Candidiasis, Chronic Mucocutaneous Candidiasis, Cutaneous Candidiasis, Oral Candidiasis, Vulvovaginal AIDS-Related Opportunistic Infections
Go PRO for all pill images
Description
Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei . Its structural formula:
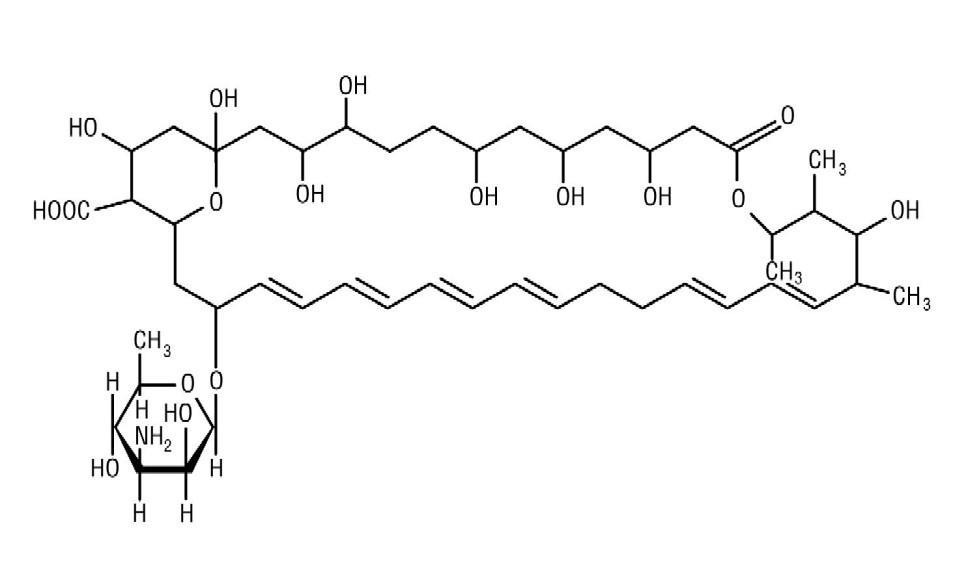
C47H75NO17Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â M.W. 926.13
Nystatin tablets are for oral administration and contain 500,000 units of nystatin per tablet.
Nystatin tablets contain the inactive ingredients: corn starch, confectioner sugar, dibasic calcium phosphate, FD&C yellow #6, FD&C red #40, FD&C blue # 2, hydroxypropyl cellulose, hypromellose, microcrystalline cellulose, magnesium stearate, polyethylene glycol, polysorbate 80, talc and titanium dioxide.
Clinical Pharmacology
Pharmacokinetics
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology
Nystatin is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. Candida albicans demonstrates no significant resistance to nystatin in vitro on repeated subculture in increasing levels of nystatin; other Candida species become quite resistant. Generally, resistance does not develop in vivo. Nystatin acts by binding to sterols in the cell membrane of susceptible Candida species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
Indications And Usage
Nystatin Tablets are intended for the treatment of non-esophageal mucus membrane gastrointestinal candidiasis.
Contraindications
Nystatin tablets are contraindicated in patients with a history of hypersensitivity to any of their components.
Precautions
General
This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. There also have been no studies to determine mutagenicity or whether this medication affects fertility in males or females.
Pregnancy
Teratogenic Effects
Category C
Animal reproduction studies have not been conducted with nystatin. It is also not known whether nystatin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether nystatin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nystatin is administered to a nursing woman.
Adverse Reactions
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported. (See PRECAUTIONS: General.)
Gastrointestinal
Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic
Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other
Tachycardia, bronchospasm, facial swelling, and nonspecific myalgia have also been rarely reported.
To report SUSPECTED ADVERSE REACTIONS, contact Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
Oral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset. There have been no reports of serious toxic effects of superinfections (see CLINICAL PHARMACOLOGY, Pharmacokinetics ).
Dosage And Administration
The usual therapeutic dosage is one to two tablets (500,000 to 1,000,000 units nystatin) three times daily. Treatment should generally be continued for at least 48 hours after clinical cure to prevent relapse.
How Supplied
Nystatin Tablets USP, 500,000 Units are round brown, film-coated tablets debossed "HP51" on one side and plain on the other side are packaged in:
            Bottles of 100: NDC 23155-051-01
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Manufactured by:
Strides Pharma Science Limited
Puducherry- 605 014, India.
PON/DRUGS/16 13 4193
Distributed by:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

1041073
Rev: 06/2020
OR
Manufactured by:
Vivimed Life Sciences Private Limited
Alathur, Kanchipuram – 603 110, Tamilnadu, India.
M.L. No.: TN00002327
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG(3784)

1043190
Revised: 08/2021
Package Label.principal Display Panel
Nystatin Tablets USP, 500,000 Units, 100 count bottles

Nystatin Tablets USP, 500,000 Units, 100 count bottles
label
label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site
![nystatin tablet, coated - (nystatin 500000 [usp'u]) image](https://pillboxwebp.pillsync.com/23155-0051-01_NLMIMAGE10_5E3C2F01.webp)

