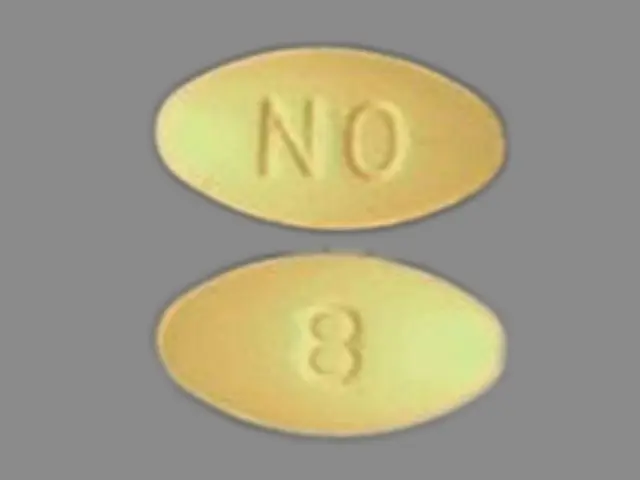
ONDANSETRON (ondansetron hydrochloride 8 mg) Dailymed
Generic: ondansetron hydrochloride is used for the treatment of Alcoholism Postoperative Nausea and Vomiting
Go PRO for all pill images
Description
The active ingredient in Ondansetron Tablets, USP is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT
receptor type. Chemically it is (¬Ī) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate.
Ondansetron HCl dihydrate is a white to off-white powder that is soluble in water and normal saline.
Each 4 mg ondansetron tablet, USP for oral administration contains ondansetron hydrochloride, USP equivalent to 4 mg of ondansetron. Each 8 mg ondansetron tablet, USP for oral administration contains ondansetron hydrochloride, USP equivalent to 8 mg of ondansetron. Each tablet also contains the inactive ingredients croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, titanium dioxide, triacetin, and iron oxide yellow (8 mg tablet only).
Product meets USP Drug Release Test 3.
Clinical Pharmacology And Clinical Trials
ALL OF THIS INFORMATION IS LOCATED AT THE MANUFACTURER'S FDA LABEL SITE:
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=834bc56a-657d-4733-9a60-47040cb5c7bf
Indications & Usage
1. Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ‚Č• 50 mg/m2.2. Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.3. Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose¬†¬†¬† fraction to the abdomen, or daily fractions to the abdomen.4. Prevention of postoperative nausea and/or vomiting. As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron tablets, USP are recommended even where the incidence of postoperative nausea and/or vomiting is low.
Contraindications
The concomitant use of apomorphine with ondansetron is contraindicated based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron.
Ondansetron tablets, USP are contraindicated for patients known to have hypersensitivity to the drug.
Warnings
Hypersensitivity reactions have been reported in patients who have exhibited hypersensitivity to other selective 5-HT
receptor antagonists.
Precautions
General
Ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distension.
Rarely and predominantly with intravenous ondansetron, transient ECG changes including QT interval prolongation have been reported.
Drug Interactions
Ondansetron does not itself appear to induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system of the liver (see
). Because ondansetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes (CYP3A4, CYP2D6, CYP1A2), inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of ondansetron. On the basis of available data, no dosage adjustment is recommended for patients on these drugs.
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, concomitant use of apomorphine with ondansetron is contraindicated.
In patients treated with potent inducers of CYP3A4 (i.e., phenytoin, carbamazepine, and rifampicin), the clearance of ondansetron was significantly increased and ondansetron blood concentrations were decreased. However, on the basis of available data, no dosage adjustment for ondansetron is recommended for patients on these drugs.
Although no pharmacokinetic drug interaction between ondansetron and tramadol has been observed, data from 2 small studies indicate that ondansetron may be associated with an increase in patient controlled administration of tramadol.
Tumor response to chemotherapy in the P-388 mouse leukemia model is not affected by ondansetron. In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
In a crossover studying 76 patients, I.V. ondansetron did not increase blood levels of high-dose methotrexate.
Use in Surgical Patients
The coadministration of ondansetron had no effect on the pharmacokinetics and pharmacodynamics of temazepam.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 and 30 mg/kg/day, respectively. Ondansetron was not mutagenic in standard tests for mutagenicity. Oral administration of ondansetron up to 15 mg/kg/day did not affect fertility or general reproductive performance of male and female rats.
Pregnancy
Pregnancy Category B. Reproduction studies have been performed in pregnant rats and rabbits at daily oral doses up to 15 and 30 mg/kg/day, respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to ondansetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Ondansetron is excreted in the breast milk of rats. It is not known whether ondansetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ondansetron is administered to a nursing woman.
Pediatric Use
Little information is available about dosage in pediatric patients 4 years of age or younger (see
sections for use in pediatric patients 4 to 18 years of age).
Geriatric Use
Of the total number of subjects enrolled in cancer chemotherapy-induced and postoperative nausea and vomiting in US- and foreign-controlled clinical trials, for which there were subgroup analyses, 938 were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment is not needed in patients over the age of 65
Adverse Reactions
The following have been reported as adverse events in clinical trials of patients treated with ondansetron, the active ingredient of ondansetron tablets, USP. A causal relationship to therapy with ondansetron tablets, USP have been unclear in many cases.
Chemotherapy-Induced Nausea and Vomiting
The adverse events in Table 5 have been reported in ‚Č• 5% of adult patients receiving a single 24-mg ondansetron tablet, USP in 2 trials. These patients were receiving concurrent highly emetogenic cisplatin-based chemotherapy regimens (cisplatin dose ‚Č• 50 mg/m).
PLEASE SEE THE COMPLETE ADVERSE REACTION INFORMATION HERE:
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=834bc56a-657d-4733-9a60-47040cb5c7bf
Overdosage
There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual intravenous doses as large as 150 mg and total daily intravenous doses as large as 252 mg have been inadvertently administered without significant adverse events. These doses are more than 10 times the recommended daily dose.
In addition to the adverse events uled above, the following events have been described in the setting of ondansetron overdose: ‚ÄúSudden blindness‚ÄĚ (amaurosis) of 2 to 3 minutes‚Äô duration plus severe constipation occurred in 1 patient that was administered 72 mg of ondansetron intravenously as a single dose. Hypotension (and faintness) occurred in a patient that took 48 mg of ondansetron tablets, USP. Following infusion of 32 mg over only a 4-minute period, a vasovagal episode with transient second-degree heart block was observed. In all instances, the events resolved completely.
Dosage & Administration
Prevention of Nausea and Vomiting Associated With Highly Emetogenic Cancer Chemotherapy
The recommended adult oral dosage of ondansetron tablets, USP is 24 mg given as three 8-mg tablets administered 30 minutes before the start of single-day highly emetogenic chemotherapy, including cisplatin ‚Č• 50 mg/m
. Multiday, single-dose administration of a 24 mg dosage has not been studied.
There is no experience with the use of a 24 mg dosage in pediatric patients.
The dosage recommendation is the same as for the general population.
Prevention of Nausea and Vomiting Associated With Moderately Emetogenic Cancer Chemotherapy
The recommended adult oral dosage is one 8-mg ondansetron tablet, USP given twice a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with a subsequent dose 8 hours after the first dose. One 8-mg ondansetron tablet, USP should be administered twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy.
For pediatric patients 12 years of age and older, the dosage is the same as for adults. For pediatric patients 4 through 11 years of age, the dosage is one 4-mg ondansetron tablet, USP or one 4-mg given 3 times a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with subsequent doses 4 and 8 hours after the first dose. One 4-mg ondansetron tablet, USP should be administered 3 times a day (every 8 hours) for 1 to 2 days after completion of chemotherapy.
The dosage is the same as for the general population.
Prevention of Nausea and Vomiting Associated With Radiotherapy, Either Total Body Irradiation, or Single High-Dose Fraction or Daily Fractions to the Abdomen
The recommended oral dosage is one 8-mg ondansetron tablet, USP given 3 times a day.
For total body irradiation, one 8-mg ondansetron tablet, USP should be administered 1 to 2 hours before each fraction of radiotherapy administered each day.
For single high-dose fraction radiotherapy to the abdomen, one 8-mg ondansetron tablet, USP should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for 1 to 2 days after completion of radiotherapy.
, one 8-mg ondansetron tablet, USP should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for each day radiotherapy is given.
There is no experience with the use of ondansetron tablet, USP in the prevention of radiation-induced nausea and vomiting in pediatric patients.
The dosage recommendation is the same as for the general population.
Postoperative Nausea and Vomiting
The recommended dosage is 16 mg given as two 8-mg ondansetron tablets, USP 1 hour before induction of anesthesia.
There is no experience with the use of ondansetron tablets, USP in the prevention of postoperative nausea and vomiting in pediatric patients.
The dosage is the same as for the general population.
Dosage Adjustment for Patients With Impaired Renal Function
The dosage recommendation is the same as for the general population. There is no experience beyond first-day administration of ondansetron.
Dosage Adjustment for Patients With Impaired Hepatic Function
In patients with severe hepatic impairment (Child-Pugh
score of 10 or greater), clearance is reduced and apparent volume of distribution is increased with a resultant increase in plasma half-life. In such patients, a total daily dose of 8 mg should not be exceeded.
Principal Display Panel
NDC: 51655-803-27
MFG: 63304-459-30
ONDANSETRON 8 MG
 12 TABLETS
RX ONLY
Lot#
Exp. Date:
Each film coated tablet contains 10 mg ondansetron hydrochloride, USP equivalent to 8 mg of ondansetron.
Dosage: See prescriber's instructions.
Store at 68-77 degrees F.
Keep out of the reach of children.
Medication guide is found at www.fda.goc/drugs/drugsafety/ucm085729
Mfg by Natco Pharma Ltd Kothur 509 228 AP India
Mfg for Ranbaxy Pharmaceuticals, Jacksonville, FL 32257 Lot#
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site