Oxistat Dailymed
Generic: oxiconazole nitrate is used for the treatment of Candidiasis, Cutaneous Tinea Pedis Tinea Versicolor Eye Infections, Fungal
Go PRO for all pill images
*Potency expressed as oxiconazole
Rx only
FOR TOPICAL DERMATOLOGIC USE ONLYÔÇö NOT FOR ORAL, OPHTHALMIC OR INTRAVAGINAL USE
Description
OXISTAT® (oxiconazole nitrate) Lotion, 1% contains the antifungal active compound oxiconazole nitrate. This formulation is for topical dermatologic use only.
Chemically, oxiconazole nitrate is 2',4'-dichloro-2-imidazol-1-ylacetophenone (Z)-[0-(2,4-dichlorobenzyl)oxime], mononitrate. The compound has the molecular formula C18H13ON3Cl4┬ĚHNO3, a molecular weight of 492.15, and the following structural formula:
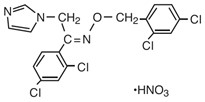
Oxiconazole nitrate is a nearly white crystalline powder, soluble in methanol; sparingly soluble in ethanol, chloroform, and acetone; and very slightly soluble in water.
OXISTAT Lotion contains 10 mg of oxiconazole per gram of lotion in a white to off-white, opaque lotion base of purified water, white petrolatum, stearyl alcohol, propylene glycol, polysorbate 60, cetyl alcohol, and benzoic acid 0.2% as a preservative.
Clinical Pharmacology
Pharmacokinetics
The penetration of oxiconazole nitrate into different layers of the skin was assessed using an in vitro permeation technique with human skin. Five hours after application of 2.5 mg/cm2 of oxiconazole nitrate cream onto human skin, the concentration of oxiconazole nitrate was demonstrated to be 16.2 ╬╝mol in the epidermis, 3.64 ╬╝mol in the upper corium, and 1.29 ╬╝mol in the deeper corium. Systemic absorption of oxiconazole nitrate is low. Using radiolabeled drug, less than 0.3% of the applied dose of oxiconazole nitrate was recovered in the urine of volunteer subjects up to 5 days after application of the cream formulation.
Neither in vitro nor in vivo studies have been conducted to establish relative activity between the lotion and cream formulations.
Microbiology
Oxiconazole nitrate is an imidazole derivative whose antifungal activity is derived primarily from the inhibition of ergosterol biosynthesis, which is critical for cellular membrane integrity. It has in vitro activity against a wide range of pathogenic fungi.
Oxiconazole has been shown to be active against most strains of the following organisms both in vitro and in clinical infections at indicated body sites (see INDICATIONS AND USAGE):
  Epidermophyton floccosum  Trichophyton mentagrophytes  Trichophyton rubrum  Malassezia furfur
The following in vitro data are available; however, their clinical significance is unknown. Oxiconazole exhibits satisfactory in vitro minimum inhibitory concentrations (MICs) against most strains of the following organisms; however, the safety and efficacy of oxiconazole in treating clinical infections due to these organisms have not been established in adequate and well-controlled clinical trials:
  Candida albicans  Microsporum audouini  Microsporum canis  Microsporum gypseum  Trichophyton tonsurans  Trichophyton violaceum
Indications And Usage
OXISTAT Lotion is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum (see DOSAGE AND ADMINISTRATION and CLINICAL STUDIES).
Contraindications
OXISTAT Lotion is contraindicated in individuals who have shown hypersensitivity to any of their components.
Warnings
OXISTAT (oxiconazole nitrate) Lotion, 1% is for topical use only and not for oral, ophthalmic or intravaginal use.
Precautions
General
OXISTAT Lotion is for external dermal use only. Avoid introduction of OXISTAT Lotion into the mouth, eyes, or vagina. If a reaction suggesting sensitivity or chemical irritation should occur with the use of OXISTAT Lotion, treatment should be discontinued and appropriate therapy instituted. If signs of epidermal irritation should occur, the drug should be discontinued.
Information for Patients
The patient should be instructed to:
1. Use OXISTAT as directed by the physician. The hands should be washed after applying the medication to the affected area(s). Avoid contact with the eyes, nose, mouth, and other mucous membranes. OXISTAT is for external use only.2. Use the medication for the full treatment time recommended by the physician, even though symptoms may have improved. Notify the physician if there is no improvement after 2 to 4 weeks, or sooner if the condition worsens (see below).3. Inform the physician if the area of application shows signs of increased irritation, itching, burning, bulering, swelling, or oozing.4. Avoid the use of occlusive dressings unless otherwise directed by the physician.5. Do not use this medication for any disorder other than that for which it was prescribed.Drug Interactions
Potential drug interactions between OXISTAT and other drugs have not been systematically evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no evidence of mutagenic effect was found in 2 mutation assays (Ames test and Chinese hamster V79 in vitro cell mutation assay) or in 2 cytogenetic assays (human peripheral blood lymphocyte in vitro chromosome aberration assay and in vivo micronucleus assay in mice).
Reproductive studies revealed no impairment of fertility in rats at oral doses of 3 mg/kg/day in females (1 time the human dose based on mg/m2) and 15 mg/kg/day in males (4 times the human dose based on mg/m2). However, at doses above this level, the following effects were observed: a reduction in the fertility parameters of males and females, a reduction in the number of sperm in vaginal smears, extended estrous cycle, and a decrease in mating frequency.
Pregnancy
Teratogenic Effects
Reproduction studies have been performed in rabbits, rats, and mice at oral doses up to 100, 150, and 200 mg/kg/day (57, 40, and 27 times the human dose based on mg/m2), respectively, and revealed no evidence of harm to the fetus due to oxiconazole nitrate. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because oxiconazole is excreted in human milk, caution should be exercised when the drug is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
A limited number of patients at or above 60 years of age (n ~ 396) have been treated with oxiconazole nitrate cream in US and non-US clinical trials, and a limited number (n = 43) have been treated with OXISTAT Lotion in US clinical trials. The number of patients is too small to permit separate analysis of efficacy and safety. No adverse events were reported with OXISTAT Lotion in geriatric patients, and the adverse reactions reported with oxiconazole nitrate cream in this population were similar to those reported by younger patients. Based on available data, no adjustment of dosage of OXISTAT Lotion in geriatric patients is warranted.
Adverse Reactions
In a controlled, multicenter clinical trial of 269 patients treated with oxiconazole nitrate lotion, 1%, 7 (2.6%) reported adverse reactions thought to be related to drug therapy. These reactions included burning and stinging (0.7% each) and pruritus, scaling, tingling, pain, and dyshidrotic eczema (0.4% each).
The following additional adverse experiences have been reported with the topical use of oxiconazole nitrate: irritation and allergic contact dermatitis, folliculitis, erythema, papules, fissure, maceration, rash, and nodules.
To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-800-308-6755 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
When 5% oxiconazole cream (5 times the concentration of the marketed product) was applied at a rate of 1 g/kg to approximately 10% of body surface area of a group of 40 male and female rats for 35 days, 3 deaths and severe dermal inflammation were reported. No overdoses in humans have been reported with use of oxiconazole nitrate cream or lotion.
Dosage And Administration
OXISTAT Lotion should be applied to affected and immediately surrounding areas once to twice daily in patients with tinea pedis, tinea corporis, or tinea cruris. Tinea corporis and tinea cruris should be treated for 2 weeks and tinea pedis for 1 month to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be reviewed.
Clinical Studies
The following definitions were applied to the clinical and microbiological outcomes in patients enrolled in the clinical trial that form the basis for the approval of OXISTAT Lotion.
Definitions
1. Mycological Cure: No evidence (culture and KOH preparation) of the baseline (original) pathogen in a specimen from the affected area taken at the 2-week post-treatment visit (for tinea [pityriasis] versicolor, mycological cure was limited to KOH only).2. Treatment Success: Both a global evaluation of 90% clinical improvement and a microbiologic eradication (see above) at the 2-week post-treatment visit.Tinea Pedis
The clinical trial for the lotion formulation line extension involved 332 evaluable patients with clinically and microbiologically established tinea pedis. Of these evaluable patients, 64% were diagnosed with hyperkeratotic plantar tinea pedis and 28% with interdigital tinea pedis. Seventy-seven percent (77%) had disease secondary to infection with Trichophyton rubrum, 18% had disease secondary to infection with Trichophyton mentagrophytes, and 4% had disease secondary to infection with Epidermophyton floccosum.
The results of this clinical trial at the 2-week post-treatment follow-up visit are shown in the following table:
Patient Outcome OXISTAT Lotion Vehicle b.i.d. q.d.
Mycological cure
67%
64%
28%
Treatment success
41%
34%
10%
In this study, the improvement and cure rates of the b.i.d.- and q.d.-treated groups did not differ significantly (95% confidence interval) from each other but were statistically (95% confidence interval) superior to the vehicle-treated group.
How Supplied
OXISTAT® (oxiconazole nitrate) Lotion, 1% is supplied in:     30-mL bottle (NDC 62559-285-30)      60-mL bottle (NDC 62559-285-60)
Store at 20┬░ to 25┬░C (68┬░ to 77┬░F); excursions permitted at 15┬░ to 30┬░C (59┬░ to 86┬░F) [see USP Controlled Room Temperature].
Shake well before using.
OXISTAT is a registered trademark of Fougera Pharmaceuticals, Inc. and is licensed to ANI Pharmaceuticals, Inc.
Distributed by:ANI Pharmaceuticals, Inc.Baudette, MN 56623
N7000 Rev 05/22
Package/label Principal Display Panel
NDC 62559-285-30 OXISTAT® (oxiconazole nitrate) Lotion, 1%* *Potency expressed as oxiconazole. For Topical Dermatologic Use ONLY.Not for Oral, Ophthalmic or Intravaginal Use. Rx only30 mL
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


