Acunol (nickel sulfate 1 [hp_x] potassium bromide 1 [hp_x] sodium bromide 1 [hp_x] zinc sulfate anhydrous 1 [hp_x] sulfur 1 [hp_x]) Dailymed
Go PRO for all pill images
Caution
Federal law prohibits dispensing without a prescription.
Description
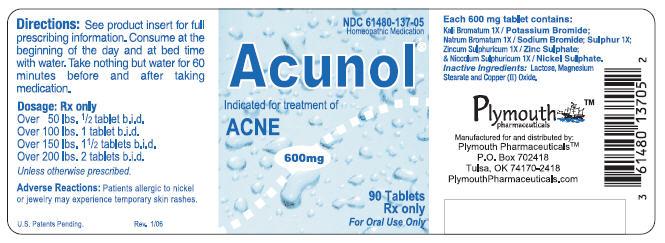
Acunolâ„¢ is a biochemical homeopathic medication indicated for the treatment of acne. 32-34 The active ingredients in each Acunolâ„¢ tablet consist of the following: Potassium Bromide (Kali Bromatum) 1X, Sodium Bromide (Natrum Bromatum) 1X, Zinc Sulphate (Zincum Sulphuricum) 1X, Sulphur 1X, and Nickel Sulphate (Niccolum Sulphuricum) 1X. These drug ingredients are uled in the Homoeopathic Pharmacopoeia of the United States (HPUS). 1
Inactive ingredients: Lactose, Magnesium Stearate, and Copper (II) Oxide.
Pharmacological class: Homeopathic drug.
Dosage form: Oral 600 mg scored tablet. May be swallowed whole, chewed or dissolved in the mouth and swallowed.
Clinical Pharmacology
The active ingredients in Acunolâ„¢ are simple biochemical compounds. The exact mechanism of action is unknown; however, it is known that bromide enhances phagocytic and bactericidal activity of neutrophils. 31
POTASSIUM BROMIDE & SODIUM BROMIDE dissolve and dissociate in the digestive tract into their ionic constituents. Each tablet contains approximately 30 mg of bromide (calculated). Ionic bromide is rapidly and completely absorbed from the intestine and distributed almost exclusively in the extracellular fluids. 7,8 Bromide is eliminated by the kidneys and the elimination half-life is 11-12 days.
ZINC SULPHATE dissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 5 mg of zinc (calculated). Zinc is an essential trace mineral necessary for protein synthesis, enzyme stimulation, and alkaline balance. 27,28 It is primarily absorbed from the duodenum and excreted via feces, urine, and sweat. 26
SULPHUR is a naturally occurring mineral that is an essential part of the human body. It exhibits anti-bacterial, anti-parasitic, fungicidal, and keratolytic properties. 26 Each tablet contains approximately 5 mg of sulphur (calculated). Sulphur is highly water soluble and as a result is easily excreted by the body via sweat and urine. 22 Since the sulphur found in Acunolâ„¢ is a naturally occurring mineral, it is radically different from sulfa drugs (sulfonamide antibiotics). Therefore, patients who are allergic to sulfa drugs CAN safely take Acunolâ„¢.
NICKEL SULPHATE dissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 0.5 mg of ionic nickel (calculated). According to studies, 15% to 50% of ionic nickel is absorbed on a fasted stomach. 2 Food markedly decreases the rate and extent of nickel absorption. 3,4 Clinical studies show that serum concentrations of nickel are variable among patients after administering the same dosage. 5 Peak serum nickel concentration is reached about two hours after oral administration. Nickel is in its highly stable divalent cation state and is therefore not expected to be metabolized to any significant degree in the body. Absorbed nickel is primarily excreted in the urine and elimination half-life is about 21 hours. 3,5 Renal clearance is rapid and efficient, and nickel does not accumulate in the body. 6
Clinical Studies
A variety of controlled clinical studies have been performed using various sources of both nickel and bromide in over 300 subjects. Clinical efficacy and safety have been documented in a significant number of subjects. Published and unpublished reports are available upon request. 9,20,21
Indications
Acunolâ„¢ is indicated for the treatment of mild to moderate acne vulgaris, acne rosacea, peri-oral dermatitis, and folliculitis. It has been found to work well with a variety of combination therapies.
Contraindications
Although there are no known contraindications, patients who are allergic to any Acunolâ„¢ ingredient should consult a physician prior to taking the medication. (Refer to Section on Hypersensitivity)
Warning
Do not use if imprinted seal under bottle cap is missing or broken. Do not use if pregnant or nursing. If allergic to nickel or metal objects such as jewelry, see PRECAUTIONS for hypersensitivity information. Lactose intolerant patients may have gastrointestinal difficulty. This has very rarely been reported at the doses used.
Precautions
Carefully adjust dosage to weight when treating young children. Use cautiously in setting of kidney disease. (see Dosage and Administration) If skin rash appears or if nervous symptoms persist, recur frequently, or are unusual, discontinue use.
Hypersensitivity
Caution should be used when administering to patients with a history of contact sensitivity to nickel (common metal exposure) or if there is a history of vesicular hand eczema (dyshidrosis, pomphylox). Nickel allergy may be confirmed by a positive nickel patch test. Most patients with hand eczema, positive nickel allergy history, or a positive nickel patch test do not have any untoward reaction to administration of Acunolâ„¢. If there is a history of nickel sensitivity or dyshidrotic hand eczema, begin with a very low dose and slowly increase to a recommended starting dose over a period of 5 weeks as tolerated, thus allowing progressive GI absorption*.
*Nickel desensitization schedule: Week Amount of Time to Take Medication Prior to Breakfast Week 1 With Breakfast Week 2 15 min Prior Week 3 30 min Prior Week 4 45 min Prior Week 5 and thereon 1 hour Prior
If new pruritic rashes occur or persist, discontinue Acunolâ„¢ and treat appropriately. Do not use if there is a history of extra-cutaneous hypersensitivity to nickel or any ingredient in Acunolâ„¢.
Information for patients
Patients using Acunolâ„¢ should receive the following information and instructions:
- This medication is to be used as directed by a physician.
- It is important to take orally at the beginning of the day on an empty stomach and at the end of the day before bed; take nothing but water for one hour afterwards to avoid interference with absorption.
Drug interactions
There are no known drug interactions. Since copper deficiency in human nutrition has occasionally been induced by supplemental zinc therapy, copper has been added in order to minimize this rare occurrence. (see Inactive ingredients) For Tetracycline or Quinolones co-administration with Acunol, see Dosage and Administration
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been done on the carcinogenesis, mutagenesis, or impairment of fertility of Acunolâ„¢. No carcinogenesis or mutagenesis has been reported in multiple animal studies for oral administration of soluble nickel and bromide salts (active ingredients) even at very high doses. 10-14
Effects of soluble potassium bromide
KBr is not uled as a carcinogen by the NTP, IARC, and OSHA. 16
Effects of sodium bromide
NaBr is not uled as a carcinogen by the IARC, NTP, or OSHA. 35
Effects of zinc sulphate
ZnSO 4 is not uled as a carcinogen by the ACGIH, IARC, NTP, or CA Prop 65. 29
Effects of sulphur
Sulphur is not uled as a carcinogen by the ACGIH, IARC, NIOSH, NTP, or OSHA. 25
Effects of soluble nickel sulphate
Studies on experimental animals have never indicated that nickel, at any dose, is a carcinogen when introduced to the body orally. Furthermore, Nickel sulphate has never been known to induce carcinogenesis via any route of introduction including: oral, inhalation, cutaneous, IM, or IP. 10-12,15 No adverse effects were noted on fertility or reproduction in a 3-generational study of albino Wistar rats fed up to 1000 ppm Ni per day, which is equivalent to 50 mg/kg per day Ni. 15
Pregnancy
Pregnancy category C
Animal reproduction studies have not been conducted with Acunolâ„¢. Acunolâ„¢ should not be given to a pregnant woman.
Nursing mothers
It is not known whether this drug is excreted in human milk. However, since many drugs are excreted in human milk, caution should be exercised when Acunolâ„¢ is administered to a nursing woman.
Pediatric use
Carefully adjust dosage to weight when treating young children.
Adverse Reactions
Acunolâ„¢ contains low doses of active ingredients. Therefore there are minimal known side effects. (see PRECAUTIONS for hypersensitivity information)
Overdosage
Bromide toxicity
Indications of toxicity due to oral overdosage of bromide may include nausea, vomiting, apathy, disturbed coordination, loss of memory, drowsiness, loss of emotional control, agitation, hallucination, tremors, depressed reflexes, stupor, and coma. Acute toxic reactions in humans have been reported at doses as low as 1000 mg. 19 This level is 33 times the dose received in one tablet of Acunolâ„¢.
Zinc sulphate toxicity
Symptoms of acute toxicity of zinc due to oral overdoseage can include dehydration, stomach pain, lethargy, dizziness, muscular incoordination, and in severe cases renal failure. 26 Chronic zinc toxicity can occur at levels of 100-300 mg/d. 30
Sulphur toxicity
The oral rat LD 50 for sulphur is reported to be greater than 5,000 mg/kg. 23 This is more than 11,000 times the maximum dose recommended for Acunolâ„¢. Ingestion of toxic levels of sulphur can cause sore throat, nausea, headache, gastrointestinal irritation, and possibly unconsciousness in severe cases. 24,25 Sulphur poses such a remote risk that is placed in the lowest toxic category possible, EPA Toxicity Category IV. 23
Nickel sulphate toxicity
The oral rat LD 50 for nickel sulphate hexahydrate is 275 mg/kg. 17 Symptoms of toxicity due to oral overdosage of nickel sulphate may include nausea, vomiting, abdominal discomfort, diarrhea, giddiness, lassitude, headaches, cough, and shortness of breath. 18 The lowest observed transitory toxic effects from human ingestion of soluble nickel salts is approximately 8 mg nickel/kg body weight. 18 This is 180 times the maximum dose recommended for Acunolâ„¢. (See below)
Dosage And Administration
Take tablets twice a day (b.i.d.) for best results. Absorption of nickel sulphate is variable among individuals. For maximum nickel absorption, tablets should be taken orally upon rising at the beginning of the day and at the end of the day before bed. Take nothing but water for one hour after taking medication to aid nickel absorption. If taking Tetracycline or Levofloxacin allow at least two hours after taking Acunol. If taking a different Quinolone antibiotic, consult manufacturer's information.
Kg lbs Starting dose Max Daily dose 23-45 51-100 ½ ∙ b.i.d. 1 ∙ b.i.d. 46-68 101-150 1 ∙ b.i.d. 2 ∙ b.i.d. 69-90 151-200 1½ ∙ b.i.d. 3 ∙ b.i.d. 91+ 201+ 2 ∙ b.i.d. 4 ∙ b.i.d.
Increase dose only if needed on a monthly basis up to the max daily dose. Treatment dose and duration depends on the individual.
In the setting of significant renal impairment
Dosage should be adjusted and serum nickel and bromide levels should be followed. Steady state trough nickel level should be drawn prior to ingesting the day's dose after one week of dosing or at appropriate intervals. Target trough serum nickel level is 10-40 mcg/L. (Caution: post dose peak levels are unreliable.) Serum bromide assay by spectrophotometry is the historic standard. 36 Concentrations higher than 50 mg/dl may be compatible with toxicity. No therapeutic target levels exist for bromide.
How Supplied
Scored tablets, off white in color with green speckles, withimprinted on one side and a score on the other side, in child-resistant and tamper-resistant bottles of 90. NDC 61480-137-05
References Section
- The Homeopathic Pharmacopoeia of the United States (HPUS), 8 th Edition, Falls Church, Virginia, 1979.
- Sunderman FW jr., Biological monitoring of nickel in humans. Scand J Work Environ Health 1993; 19 suppl 1:34-38.
- Sunderman FW jr., Hopfer SM, Sweeny KR, Marcus AH, Most BM, Creason J. Nickel absorption and kinetics in human volunteers, P.S.E.B.M. 1989; 191:5-11.
- Solomons NW, Viteri F, Shuler TR, Nielsen FH. Bioavailability of nickel in man: Effects of foods and chemically-defined dietary constituents on the absorption of inorganic nickel. J Nutr 1982; (2); 112; 39-50.
- Christensen OB, lagesson V. Nickel concentration of blood and urine after oral administration. Annals of Clinical and Laboratory Science 1981;2(2);119-125.
- Nielsen FH. Is nickel nutritionally important? Nutrition Today 1993;28(1):14-19.
- Vaiseman N, Koren G, Pencharz P. Pharmacokinetics or oral and intravenous bromide in normal volunteers. Clinical Toxicology 1986;24(5):403-413.
- Van Leeuwen FXR, Sangster B. The toxicology of bromide ion. CRC Critical Reviews in Toxicilogy 1987; 18(3):189-213.
- Smith SA. Oral supplementation of nickel and bromide in psoriasis vulgaris using nickel sulfate and sodium bromide. 1995 (unpublished report).
- Miller MJ, Bogdan KG, Leach JF, and Gray AJ. Ambient air criteria document, Bureau of Toxic Substance Assessment, New York Department of Health, Albany, NY 1989.
- US EPA, Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, health assessment document for nickel and nickel compounds. Washington DC, EPA/600/8-83/012FF.
- US EPA, Environmental Criteria and Assessment Office, Office of health and Environmental Assessment. Drinking water quantification of toxicological effects for nickel. ECAO-CIN-443. 1991.
- US FDA, Center for Food Safety and Applied Nutrition. Guidance document for nickel in shellfish. 1993.
- Buselmaier W. von, Rohrborn G, and Propping G. Mutagenitats – Untersuchugen mit Pestiziden im Host – Mediated Assay und mit dem Dominanten Letaltest an der Maus. Biol Zbl 1972; 91:311-325.
- Ambrose AM, Larson PS, Borzelleca JF, and Hennigar GR, Jr. Long term toxicologic assessment of nickel in rats and dogs. Journal of Food Science and Technology 1976;13:181-187.
- MSDS Sheet No. 247 Potassium Bromide. Schenectady, NY: Genium Publishing Corp 1991.
- MSDS Sheet No. 37 Nickel Sulfate. Schenectady, NY: Genium Publishing Corp 1993.
- Sunderman FW Jr., Dingle B, Hopfer SM, and Swift T. Acute nickel toxicity in electroplating workers who accidentally ingested a solution of nickel sulfate and nickel chloride. American Journal of Industrial Medicine 1988;14;257- 266.
- Martindale: The Extra Pharmacopoeia 27 th ed. Wade A, editor. The Pharmaceutical Press: London, 1977, pp273-274.
- Smith SA, et al, Improvement of Psoriasis Vulgaris with Oral Nickel Dibromide. Archives of Dermatology 1997; 133:661-663.
- Smith SA, Baker AE, etal. Effective Treatment of Seborrheic Dermatitis Using a Low Dose, Oral Homeopathic Medication... Alt Med Rev 2002; 7, pp59-67.
- MSM- The Wonder Supplement of the Millennium. <http://www.eftel.com.au/~mcs/msm/msm> 7 July 2005.
- Pesticide Information Profile: Sulfur. <http://pmep.cce.cornell.edu/profiles/extoxnet/pyrethrins-ziram/sulfurext.. html> 7 July 2005.
- MSDS Number 58138: Sulfur. 12 Feb. 2004 Mallinckrodt Baker, Inc. 7 July 2005 <http://www.jtbaker.cvom/msds/englishhtml/s8138.htm>.
- MSDS Sulfur: Hazardous According to Criteria of Worksafe Australia. Dec. 1998. Seton Compliance Resource Center <http://www.setonresource.center.com>.
- Remington: The Science and Practice of Pharmacy. 19 th Edition, Mack Publishing Company, 1995.
- Zinc.T.J.Clark.19July2005.<http://www.mailguard.org/MINERALS/zinc.htm>.
- The Benefits of Zinc. Dr. George Obikoya. 2004. The Vitamins & Nutrition Center. 19 July 2005 <http://www.vitamins-nutrition.org/vitamins/zinc.html>.
- Material Safety Data Sheet: Zinc sulfate. 11/18/2004. Fisher Scientific. 19 Dec. 2005 <http://fscimage.fishersci.com/msds/89980.htm>.
- Zinc. Pharmavite ® LLC. 2003-2005. Vitamin & Herb University. 19 July 2005. <http://www.vitaminherbuniversity.com/topic.asp?categoryid=2&topicid=1029>.
- Steele RW, Woody RC. Enhanced Neutrophil Function in Children on Bromide Therapy. American Journal of Medical Sciences 1991; 302(3): 145 – 147.
- Reckeweg, Hans-Heinrich, Materia Medica, 1983, first English edition.
- Boericke, William, Materia Medica with Reperatory, 1927, ninth edition.
- Clarke, John Henry, A Dictionary of Practical Materia Medica, 1921, reprint edition 1996.
- MSDS Sheet No. 743 Sodium Bromide. Schenectady, NY: Genium Publishing Corp 1991.
- Sonnenwirth AC, Jarett L, eds. Bromide. In: Gradwohl's Clinical Laboratory Methods and Diagnosis, 1980; Vol. 1, Ch. 19, Analytical Toxicology, 401-402.
RxSales@PlymouthPharmaceuticals.com
www.PlymouthPharmaceuticals.com
Plymouth Pharmaceuticals Inc. dba LOMA LUX Laboratories;Â Â Â P.O. BOX 702418;Â Â Â Tulsa, OK 74170-2418
Phone 800.316.9636,   918.664.9882,   Fax 918.664.9884
Revised 09.13.06 TDS
Principal Display Panel - 90 Tablet Label
NDC 61480-137-05 Homeopathic Medication
Acunol ®
Indicated for treatment of ACNE
600mg
90 Tablets Rx only For Oral Use Only
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site
![Acunol tablet - (nickel sulfate 1 [hp_x] potassium bromide 1 [hp_x] sodium bromide 1 [hp_x] zinc sulfate anhydrous 1 [hp_x] sulfur 1 [hp_x]) image](https://pillboxwebp.pillsync.com/capsule.webp)

