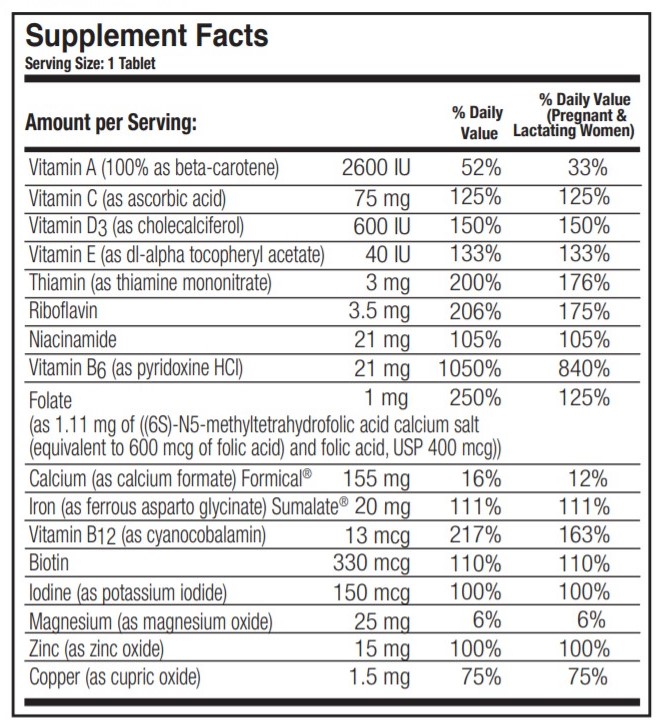
Prenate Elite (.beta.-carotene 2600 [iu] ascorbic acid 75 mg cholecalciferol 600 [iu] .alpha.-tocopherol acetate, dl- 40 [iu] thiamine mononitrate 3 mg riboflavin 3.5 mg niacinamide 21 mg pyridoxine hydrochloride 21 mg folic acid 400 ug 5-methyltetrahydrofolic acid 600 ug calcium formate 155 mg ferrous asparto glycinate 20 mg cyanocobalamin 13 ug biotin 330 ug potassium iodide 150 ug magnesium oxide 25 mg zinc oxide 15 mg cupric oxide 1.5 mg) Dailymed
Go PRO for all pill images
Rx Only Dietary Supplement
Description Section
DESCRIPTION: PRENATE ELITE ® is a white, oval, oil- and water-soluble, multivitamin/multimineral, film-coated tablet debossed with “PN” on one side and blank on the other.
Inactive Ingredient Section
OTHER INGREDIENTS: Microcrystalline Cellulose, Silicon Dioxide, Croscarmellose Sodium, Stearic Acid, Magnesium Stearate, Film Coating (Hydroxypropyl Methylcellulose, Triacetin, Titanium Dioxide).
Indications & Usage Section
INDICATIONS: PRENATE ELITE ® is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
Contraindications Section
CONTRAINDICATIONS: PRENATE ELITE ® is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Precautions Section
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B 12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Boxed Warning Section
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Adverse Reactions Section
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Dosage & Administration Section
DOSAGE AND ADMINISTRATION: Before, during and/or after pregnancy, one tablet daily or as directed by a physician.
How Supplied Section
HOW SUPPLIED: Bottles of 30 tablets (75854-314-30). The uled product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
Storage And Handling Section
Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
MANUFACTURED FOR: Avion Pharmaceuticals, LLC Alpharetta, Georgia 30005 1-888-61-AVION
Rev. 0519-01
Formical ® is a registered trademark of Nephro-Tech 1, LLC, covered by one or more claims of U.S. Patent No. 6,528,542.
Sumalate ® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 5,516,925, 6,716,814, 8,007,846, and 8,425,956.
Package Label.principal Display Panel
75854-314-30
Prenate Elite ® PRENATAL VITAMINS-FILM-COATED TABLETS
GLUTEN FREE Rx Only Dietary Supplement
30 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site
![Prenate Elite tablet, coated - (.beta.-carotene 2600 [iu] ascorbic acid 75 mg cholecalciferol 600 [iu] .alpha.-tocopherol acetate, dl- 40 [iu] thiamine mononitrate 3 mg riboflavin 3.5 mg niacinamide 21 mg pyridoxine hydrochloride 21 mg folic acid 400 ug 5-methyltetrahydrofolic acid 600 ug calcium formate 155 mg ferrous asparto glycinate 20 mg cyanocobalamin 13 ug biotin 330 ug potassium iodide 150 ug magnesium oxide 25 mg zinc oxide 15 mg cupric oxide 1.5 mg) image](https://pillboxwebp.pillsync.com/capsule.webp)

