Phentermine Hydrochloride (phentermine hydrochloride 37.5 mg) Dailymed
Generic: phentermine hydrochloride is used for the treatment of Breast Feeding Cardiovascular Diseases Glaucoma Hyperthyroidism Obesity Pregnancy Psychomotor Agitation Substance-Related Disorders Arteriosclerosis Child Hypertension Substance Abuse, Intravenous
IMPRINT: MP 273
SHAPE: oval
COLOR: white SCORE: 2
All Imprints
phentermine hydrochloride 37.5 mg - mp 273 oval white
phentermine hydrochloride 37.5 mg oral tablet - mp 273 oval white
Go PRO for all pill images
Indications & Usage
Phentermine hydrochloride tablets USP are indicated as a short-term (a few weeks) adjunct in a regimen
of weight reduction based on exercise, behavioral modification and caloric restriction in the
management of exogenous obesity for patients with an initial body mass index greater than or equal to
30 kg/m , or greater than or equal to 27 kg/m in the presence of other risk factors (e.g., controlled
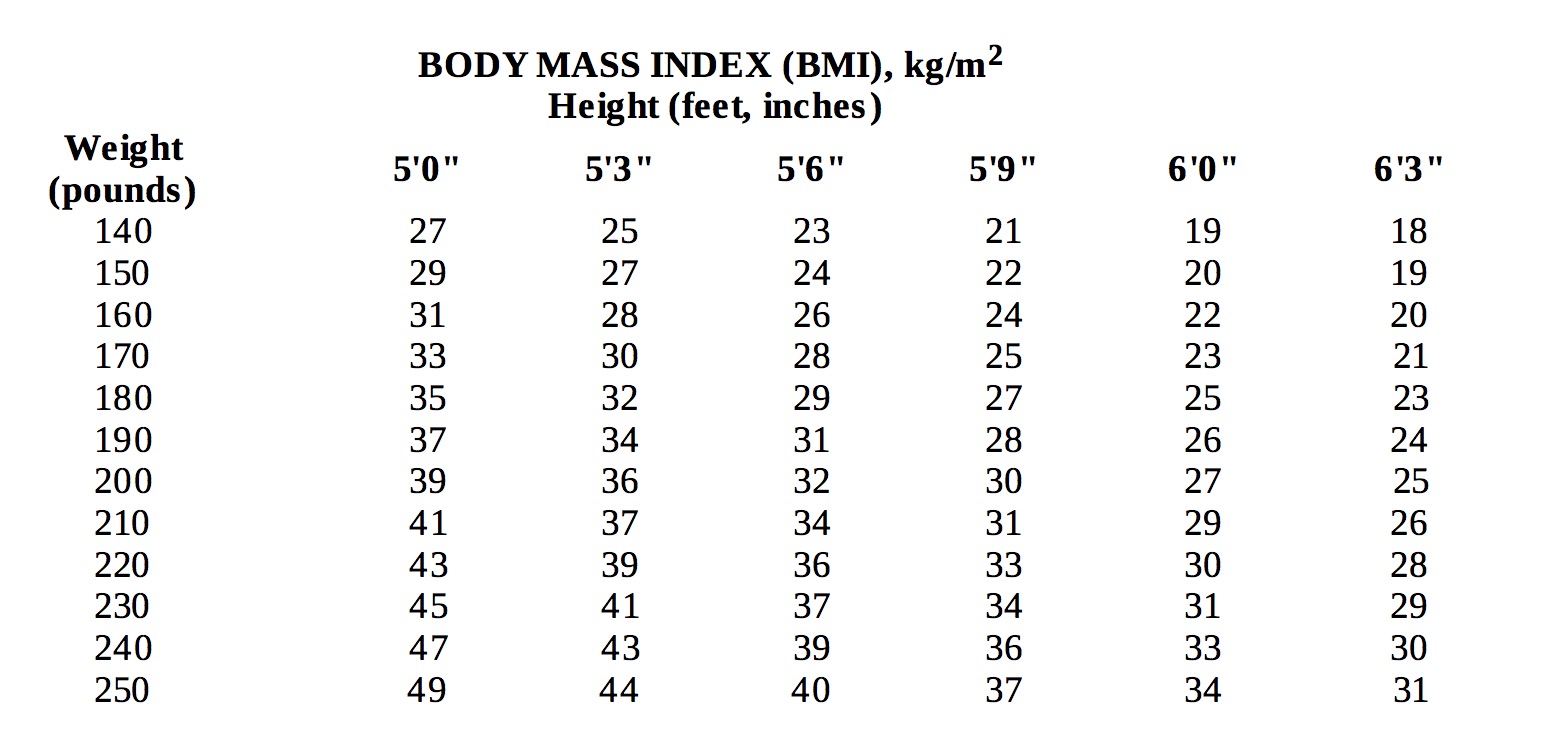
hypertension, diabetes, hyperlipidemia). Below is a chart of body mass index (BMI) based on various heights and weights.
BMI is calculated by taking the patient's weight, in kilograms (kg), divided by the patient's height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
Description
Phentermine hydrochloride USP is a sympathomimetic amine anorectic. Its chemical name is a,a- dimethylphenethylamine hydrochloride. The structural formula is as follows:
Phentermine hydrochloride USP is a white, odorless, hygroscopic, crystalline powder which is soluble in water and lower alcohols, slightly soluble in chloroform and insoluble in ether.
Phentermine hydrochloride tablets USP are available as an oral tablet containing 37.5 mg of phentermine hydrochloride USP (equivalent to 30 mg of phentermine base). Each phentermine hydrochloride tablet USP also contains the inactive ingredients microcrystalline cellulose, pregelatinized starch, anhydrous lactose, crospovidone, colloidal silicon dioxide, magnesium stearate, sucrose, corn starch and FD&C Blue #1.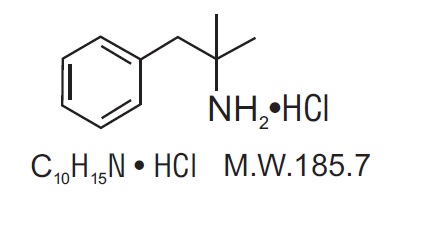
Package And Display
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



