Seysara (sarecycline hydrochloride 150 mg) Dailymed
Generic: sarecycline hydrochloride is used for the treatment of Acne Vulgaris
Go PRO for all pill images
1 Indications And Usage
SEYSARA® (sarecycline) tablet, is indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older.
Limitations of Use Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections [see Clinical Studies (14)].
To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SEYSARA should be used only as indicated [see Warnings and Precautions (5.6)].
SEYSARA® is a tetracycline-class drug indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older. (1 )
Limitations of Use Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SEYSARA should be used only as indicated [see Warnings and Precautions (5.6 )].
2 Dosage And Administration
The recommended dosage of SEYSARA is based on body weight described in Table 1. If there is no improvement after 12 weeks, reassess treatment with SEYSARA.
Table 1: Dosing Table for SEYSARA Body Weight (kg) Tablet Strength 33 to 54 kg 60 mg tablet 55 to 84 kg 100 mg tablet 85 to 136 kg 150 mg tablet
Take SEYSARA once daily, with or without food. To reduce the risk of esophageal irritation and ulceration, administer SEYSARA with adequate amounts of fluid.
The recommended dosage of SEYSARA is once daily with or without food (2 ):
- 60 mg for patients who weigh 33-54 kg,
- 100 mg for patients who weigh 55-84 kg,
- 150 mg for patients who weigh 85-136 kg.
3 Dosage Forms And Strengths
SEYSARA (sarecycline) tablets:
- 60 mg: capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side.
- 100 mg: capsule-shaped, yellow, film-coated tablets debossed with “S100” on one side and blank on the other side.
- 150 mg: capsule-shaped, yellow, film-coated tablets debossed with “S150” on one side and blank on the other side.
Tablets: 60 mg, 100 mg, 150 mg (3 )
4 Contraindications
SEYSARA is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
SEYSARA is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines. (4 )
5 Warnings And Precautions
- The use of SEYSARA during tooth development (second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). (
5.1 )- If Clostridium difficile Associated Diarrhea (antibiotic associated colitis) occurs, discontinue SEYSARA. (
5.2 )- Central nervous system side effects, including light-headedness, dizziness or vertigo, have been reported with tetracycline use. Patients who experience these symptoms should be cautioned about driving vehicles or using hazardous machinery. These symptoms may disappear during therapy and may disappear when the drug is discontinued. (
5.3 )- SEYSARA may cause intracranial hypertension. Discontinue SEYSARA if symptoms occur. (
5.4 )- Photosensitivity can occur with SEYSARA. Patients should minimize or avoid exposure to natural or artificial sunlight. (
5.5 )5.1 Teratogenic Effects
SEYSARA, like other tetracyclines, can cause fetal harm when administered to a pregnant woman. If SEYSARA is used during pregnancy or if the patient becomes pregnant while taking SEYSARA, the patient should be informed of the potential hazard to the fetus and treatment should be stopped immediately.
The use of drugs of the tetracycline-class during tooth development (second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of these drugs, but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported.
All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued. Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development on the developing fetus. Evidence of embryotoxicity has been noted in animals treated with SEYSARA during pregnancy in association with maternal toxicity [see Use in Specific Populations (8.1)].
5.2 Associated Diarrhea (Antibiotic Associated Colitis)
Clostridium difficile associated diarrhea (CDAD) has been reported with nearly all antibacterial agents, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to potential overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile should be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Central Nervous System Effects
Central nervous system side effects including light-headedness, dizziness or vertigo have been reported with tetracycline use. Patients who experience these symptoms should be cautioned about driving vehicles or using hazardous machinery. These symptoms may disappear during therapy and may disappear when the drug is discontinued.
5.4 Intracranial Hypertension
Intracranial hypertension in adults and adolescents has been associated with the use of tetracyclines. Clinical manifestations include headache, blurred vision and papilledema. Although signs and symptoms of intracranial hypertension resolve after discontinuation of treatment, the possibility for sequelae such as visual loss that may be permanent or severe exists. Women of childbearing age who are overweight have a greater risk for developing intracranial hypertension. Patients should be questioned for visual disturbances prior to initiation of treatment with tetracyclines. Concomitant use of isotretinoin and SEYSARA should be avoided because isotretinoin, a systemic retinoid, is also known to cause intracranial hypertension [see Drug Interactions (7.1)]. If visual disturbance occurs during treatment, patients should be checked for papilledema.
5.5 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using SEYSARA. If patients need to be outdoors while using SEYSARA, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician.
5.6 Development of Drug Resistant Bacteria
Bacterial resistance to tetracyclines may develop in patients using SEYSARA. Because of the potential for drug-resistant bacteria to develop during the use of SEYSARA, it should only be used as indicated.
5.7 Superinfection/Potential for Microbial Overgrowth
As with other antibiotic preparations, use of SEYSARA may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, SEYSARA should be discontinued and appropriate therapy instituted.
6 Adverse Reactions
Most common adverse reaction (incidence ≥ 1%) is nausea. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Almirall at 1-866-665-2782 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 1064 subjects and 1069 subjects with moderate to severe acne vulgaris were treated with SEYSARA and placebo, respectively, for 12 weeks in 3 controlled clinical trials. The only adverse drug reaction that was reported in at least 1% of subjects was nausea, SEYSARA (3.1%) versus placebo (2.0%).
The following additional adverse drug reactions occurred in less than 1% of female SEYSARA subjects: vulvovaginal mycotic infection (0.8%) and vulvovaginal candidiasis (0.6%).
7 Drug Interactions
- Oral retinoids: avoid coadministration. (
5.4 ,7.1 )- Antacids and iron preparations: separate dosing of SEYSARA. (
7.1 )- Penicillin: avoid coadministration. (
7.2 )- Anticoagulants: decrease anticoagulant dosage as appropriate. (
7.2 )- P-glycoprotein substrates: monitor for toxicities of drugs that may require dosage reduction. (
7.2 )7.1 Effect of Other Drugs on SEYSARA
Oral Retinoids
Tetracyclines may cause increased intracranial pressure as do oral retinoids, including isotretinoin and acitretin [see Warnings and Precautions (5.4)]. Avoid coadministration of SEYSARA with oral retinoids.
Antacids and Iron Preparations
Coadministration with antacids containing aluminum, calcium or magnesium, bismuth subsalicylate, and iron-containing preparations may impair absorption of SEYSARA, similar to other tetracyclines, which may decrease its efficacy. Separate dosing of SEYSARA from antacids containing aluminum, calcium or magnesium, bismuth subsalicylate, and iron-containing preparations.
7.2 Effect of SEYSARA on Other Drugs
Penicillin
Similar to other tetracyclines, SEYSARA may interfere with the bactericidal action of penicillin. Avoid coadministration of SEYSARA with penicillin.
Anticoagulants
Similar to other tetracyclines, SEYSARA may depress plasma prothrombin activity, which may increase the risk of bleeding in patients who are on anticoagulant therapy. Decrease anticoagulant dosage when coadministered with SEYSARA as appropriate.
P-Glycoprotein (P-gp) Substrates
Concomitant use of SEYSARA may increase concentrations of concomitantly administered P-gp substrates (e.g. digoxin). Monitor for toxicities of drugs that are P-gp substrates and may require dosage reduction when given concurrently with SEYSARA [see Clinical Pharmacology (12.3)].
Oral Hormonal Contraceptives
There is no clinically significant effect of SEYSARA on the efficacy of oral contraceptives containing ethinyl estradiol and norethindrone acetate [see Clinical Pharmacology (12.3)].
8 Use In Specific Populations
- Sarecycline, like other tetracycline-class drugs, can cause fetal harm when administered to a pregnant woman. (
5.1 ,8.1 )- The use of drugs of the tetracycline class during tooth development may cause permanent discoloration of teeth. (
5.1 ,8.4 )- Lactation: Breastfeeding is not recommended. (
8.2 )8.1 Pregnancy
Risk Summary
SEYSARA, like tetracycline class drugs, may cause fetal harm, permanent discoloration of teeth, and reversible inhibition of bone growth when administered during pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]. The limited available human data are not sufficient to inform a drug-associated risk for birth defects or miscarriage. Tetracyclines are known to cross the placental barrier; therefore, SEYSARA may be transmitted from the mother to the developing fetus. In animal reproduction studies, sarecycline induced skeletal malformations in fetuses when orally administered to pregnant rats during the period of organogenesis at a dose 1.4 times the maximum recommended human dose (MRHD) of 150 mg/day (based on AUC comparison). When dosing with sarecycline continued through the period of lactation, decreases in offspring survival, offspring body weight, and implantation sites and viable embryos in offspring females occurred at a dose 3 times the MRHD (based on AUC comparison) [see Data]. The potential risk to the fetus outweighs the potential benefit to the mother from SEYSARA use during pregnancy; therefore, pregnant patients should discontinue SEYSARA as soon as pregnancy is recognized.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryofetal developmental study in rats, sarecycline was administered to pregnant rats at oral doses up to 500 mg/kg/day during the period of organogenesis. Decreases in maternal body weight, fetal body weight and litter size and increases in the number of resorption and postimplantation loss occurred at 500 mg/kg/day (7 times the MRHD based on AUC comparison). Skeletal malformations (bent forelimb, hindlimb, and scapula) occurred at all dose levels (≥ 50 mg/kg/day, 1.4 times the MRHD based on AUC comparison).
In an embryofetal developmental study in rabbits, sarecycline was administered to pregnant rabbits at oral doses up to 150 mg/kg/day during the period of organogenesis. Excessive maternal toxicity (mortality/moribundity/abortion) occurred at 150 mg/kg/day (5 times the MRHD based on AUC comparison) and this dose group was terminated early. Maternal moribundity also occurred at 100 mg/kg/day (0.6 times the MRHD based on AUC comparison). No significant embryofetal toxicity or malformations were observed at doses up to 100 mg/kg/day (0.6 times the MRHD based on AUC comparison).
In a pre- and post-natal developmental study in rats, sarecycline was administered to maternal rats at oral doses up to 400 mg/kg/day during the period of organogenesis through lactation. Excessive litter toxicity (litter loss and stillbirth) occurred at 400 mg/kg/day (8 times the MRHD based on AUC comparison), which led to early termination of dams at parturition. Decreases in body weight and food consumption of dams during the lactation period occurred at 150 mg/kg/day (3 times the MRHD based on AUC comparison). Decreases in offspring survival and offspring body weight during the preweaning and growth period, and decreases in implantation sites and viable embryos in female offspring occurred at 150 mg/kg/day (3 times the MRHD based on AUC comparison). No significant maternal or developmental toxicity was observed at 50 mg/kg/day (1.4 times the MRHD based on AUC comparison).
8.2 Lactation
Risk Summary
Tetracyclines are excreted in human milk. Because of the potential for serious adverse reactions on bone and tooth development in nursing infants from tetracycline-class antibiotics, advise a woman that breastfeeding is not recommended with SEYSARA therapy [see Warnings and Precautions (5.1)].
8.3 Females and Males of Reproductive Potential
Infertility
Avoid using SEYSARA in males who are attempting to conceive a child. In a fertility study in rats, sarecycline adversely affected spermatogenesis when orally administered to male rats at a dose 8 times the MRHD (based on AUC comparison) [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of SEYSARA have been established in pediatric patients 9 years of age and older for the treatment of moderate to severe inflammatory lesions of non-nodular acne vulgaris [see Pharmacokinetics (12.3) and Clinical Studies (14)].
Safety and effectiveness of SEYSARA in pediatric patients below the age of 9 years has not been established. Use of tetracycline-class antibiotics below the age of 8 is not recommended due to the potential for tooth discoloration [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of SEYSARA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10 Overdosage
In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases of overdose.
11 Description
SEYSARA (sarecycline) tablets are a tetracycline class drug for oral administration. Sarecycline hydrochloride is chemically described as (4S,4aS,5aR,12aS)-4-(dimethylamino)-3,10,12,12a-tetrahydroxy-7-[(methoxy-(methyl)-amino)- methyl]-1,11-dioxo-1,4,4a,5,5a,6,11, 12a-octahydrotetracene-2-carboxamide monohydrochloride with an empirical formula of C24H29N3O8.HCl and a molecular weight of 523.96.
The structural formula is represented below:
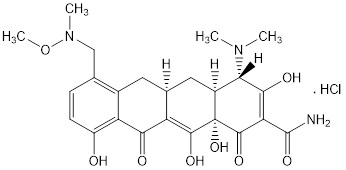
SEYSARA tablets contain 64.5 mg, 107.5 mg, and 161.2 mg of sarecycline hydrochloride equivalent to 60 mg, 100 mg, and 150 mg sarecycline respectively. Inactive ingredients in the tablet formulations are: microcrystalline cellulose, povidone, sodium starch glycolate, and sodium stearyl fumarate. The yellow film coating contains D&C yellow #10 aluminum lake, iron oxide yellow, methacrylic acid copolymer type C, polyethylene glycol, polyvinyl alcohol, sodium bicarbonate, talc, and titatnium dioxide.
12 Clinical Pharmacology
12.1 Mechanism of Action
Sarecycline is an aminomethylcycline within the tetracycline class of drugs. [see CLINICAL PHARMACOLOGY (12.4)]. The mechanism of action of SEYSARA in treating the inflammatory lesions of non-nodular acne vulgaris is not known.
12.2 Pharmacodynamics
The pharmacodynamics of SEYSARA for the treatment of acne vulgaris are unknown.
Cardiac Electrophysiology
At approximately 3 times the maximum recommended dose, SEYSARA did not prolong the QT interval to a clinically relevant extent.
12.3 Pharmacokinetics
Increasing the SEYSARA dose from 60 to 150 mg once daily in healthy subjects resulted in a slightly less than proportional increase in sarcyeline steady-state Cmax and AUCtau. A mean accumulation ratio of sarecycline ranges from 1.5 to 1.6 fold with repeated dosing. Steady-state of sarecycline was reached by Day 7.
Absorption
The median time to peak plasma concentration (Tmax) of sarecycline is 1.5 to 2.0 hours.
Effect of Food
Coadministration with a high-fat (approximately 50% of total caloric content of the meal), high-calorie (800 to 1000 Kcal) meal that included milk delayed Tmax by approximately 0.53 hour and decreased sarecycline Cmax by 31% and AUC by 27%.
Distribution
Protein binding of sarecycline is 62.5% to 74.7% in vitro. The mean apparent volume of distribution of sarecycline at steady-state ranges from 91.4 L to 97.0 L.
Elimination
The mean apparent oral clearance (CL/F) of sarecycline at steady state is approximately 3 L/h. The mean elimination half-life of sarecycline is 21 to 22 hours.
Metabolism
Metabolism of sarecycline by enzymes in human liver microsomes is minimal (< 15%) in vitro. Minor metabolites resulting from non-enzymic epimerization, O-/N-demethylation, hydroxylation, and desaturation have been found.
Excretion
After a single 100 mg oral dose of radiolabeled sarecycline, 42.6% of the dose was recovered in feces (14.9% as unchanged) and 44.1% in urine (24.7% as unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of sarecycline were observed based on age (11 to 73 years), weight (42 to 133 kg), sex, renal impairment, or mild to moderate hepatic impairment (Child Pugh A to B). The effect of end-stage renal disease (ESRD) or severe hepatic impairment (Child-Pugh C) on sarecycline pharmacokinetics has not been assessed.
Drug Interaction Studies
Clinical Studies
Coadministration of SEYSARA with a combination oral contraceptive, ethinyl estradiol (EE) 20 mcg plus norethindrone (NE) acetate 1 mg, increased EE Cmax by 14% and AUCtau by 11%, and increased NE Cmax by 18% and AUCtau by 23%.
Coadministration of a single dose of SEYSARA 150 mg resulted in a 26% increase in Cmax of digoxin, a P-gp substrate.
In Vitro Studies
Sarecycline is not a substrate for P-gp, BCRP, OATP1B1, or OATP1B3.
Sarecycline is a P-gp inhibitor. Sarecycline does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4/5 isozymes, and does not inhibit OATP1B1, OATP1B3, OCT2, OAT1, OAT3, or BCRP.
Sarecycline does not induce CYP1A2, CYP2B6, or CYP3A4/5 isozymes.
12.4 Microbiology
Mechanism of Action In P. acnes, sarecycline binds to the 30S ribosomal subunit and interacts with 16S ribosomal RNA. Furthermore, it protrudes its C7 moiety into the mRNA binding channel to interact with mRNA. Sarecycline blocks P. acnes protein synthesis and inhibits bacterial growth however, the clinical significance is unknown.
Resistance P. acnes strains displayed a low propensity for the development of resistance to sarecycline, with spontaneous mutation frequencies being 10-10 at 4 – 8 × MIC.
Antimicrobial Activity Sarecycline is active in vitro against most isolates of Propionibacterium acnes.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year oral mouse carcinogenicity study and a 2-year oral rat carcinogenicity study, no drug-related neoplasms were observed in male mice at oral doses of sarecycline up to 100 mg/kg/day (approximately equal to the MRHD based on AUC comparison) or in female mice at doses up to 60 mg/kg/day (approximately equal to the MRHD based on AUC comparison), or in rats at doses up to 200/100 mg/kg/day (dose reduced from 200 to 100 mg/kg/day due to increased mortality; 8 times the MRHD based on AUC comparison).
Sarecycline was not mutagenic or clastogenic in a series of in vitro and in vivo genotoxicity studies, including a bacteria reverse mutation (Ames) assay, an in vitro chromosomal aberration assay in CHO cells, the L5178Y/TK+/- Mouse Lymphoma Assay, and an in vivo micronucleus assay in rats.
In a fertility and early embryonic development study in rats, sarecycline was administered to both male and female rats at oral doses up to 400 mg/kg/day prior to pairing and through the mating and postmating period. Female fertility was not affected at doses up to 400 mg/kg/day (8 times the MRHD based on AUC comparison). In sperm evaluation, decreased sperm motility, decreased sperm count and concentration, and an increase in percent abnormal sperm occurred at 400 mg/kg/day (8 times the MRHD based on AUC comparison). Male fertility was not affected at doses up to 150 mg/kg/day (4 times the MRHD based on AUC comparison).
14 Clinical Studies
The safety and efficacy of once daily SEYSARA was assessed in two 12-week multicenter, randomized, double-blind, placebo-controlled studies (Study 1 [NCT02320149] and Study 2 [NCT02322866]). Efficacy was assessed in a total of 2002 subjects 9 years of age and older. Overall, 57% were female, 78% were Caucasian, 15% were Black or African American and 51% were adults (18 to 45 years of age). Subjects were randomized to receive either SEYSARA or placebo once daily.
The two co-primary efficacy endpoints were:
- Percentage of subjects with Investigator’s Global Assessment (IGA) success: a score of clear (0) or almost clear (1) and 2-point decrease from baseline on IGA score at Week 12.
- Absolute reduction from baseline in inflammatory lesion counts at Week 12.
The results at Week 12 are presented in the following table.
Table 2: Clinical Efficacy of SEYSARA at Week 12 Study 1 Study 2 SEYSARA (N=483) Placebo (N=485) SEYSARA (N=519) Placebo (N=515) Investigator’s Global Assessment IGA Success 21.9% 10.5% 22.6% 15.3% Inflammatory Lesions Mean absolute reduction 15.3 10.2 15.5 11.1 Mean percent reduction 52.2% 35.2% 50.8% 36.4%
Mean absolute and percent reduction in inflammatory lesions was also greater with SEYSARA compared to placebo at Weeks 3, 6, and 9 for both studies.
16 How Supplied/storage And Handling
How Supplied
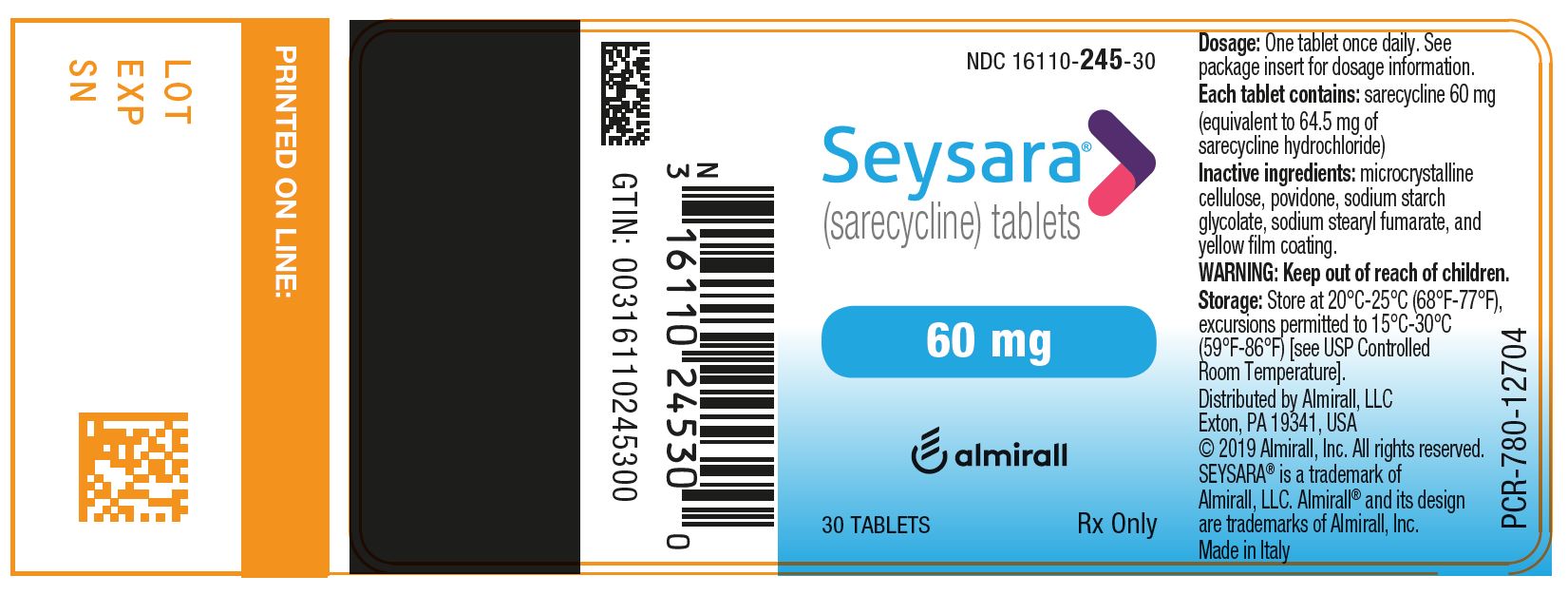
1) SEYSARA (sarecycline) tablets, 60 mg are capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side.
- Bottles of 30 tablets with child-resistant closure: NDC: 16110-245-30
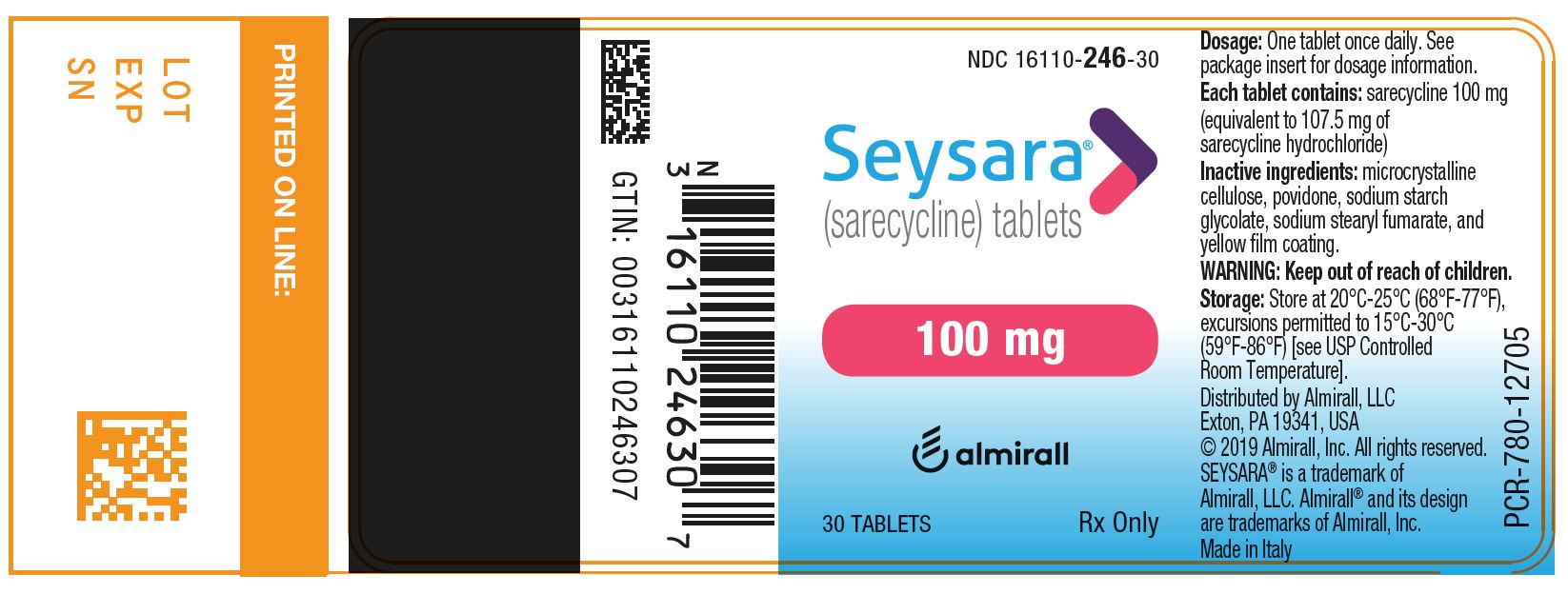
2) SEYSARA (sarecycline) tablets, 100 mg are capsule-shaped, yellow, film-coated tablets debossed with “S100” on one side and blank on the other side.
- Bottles of 30 tablets with child-resistant closure: NDC: 16110-246-30
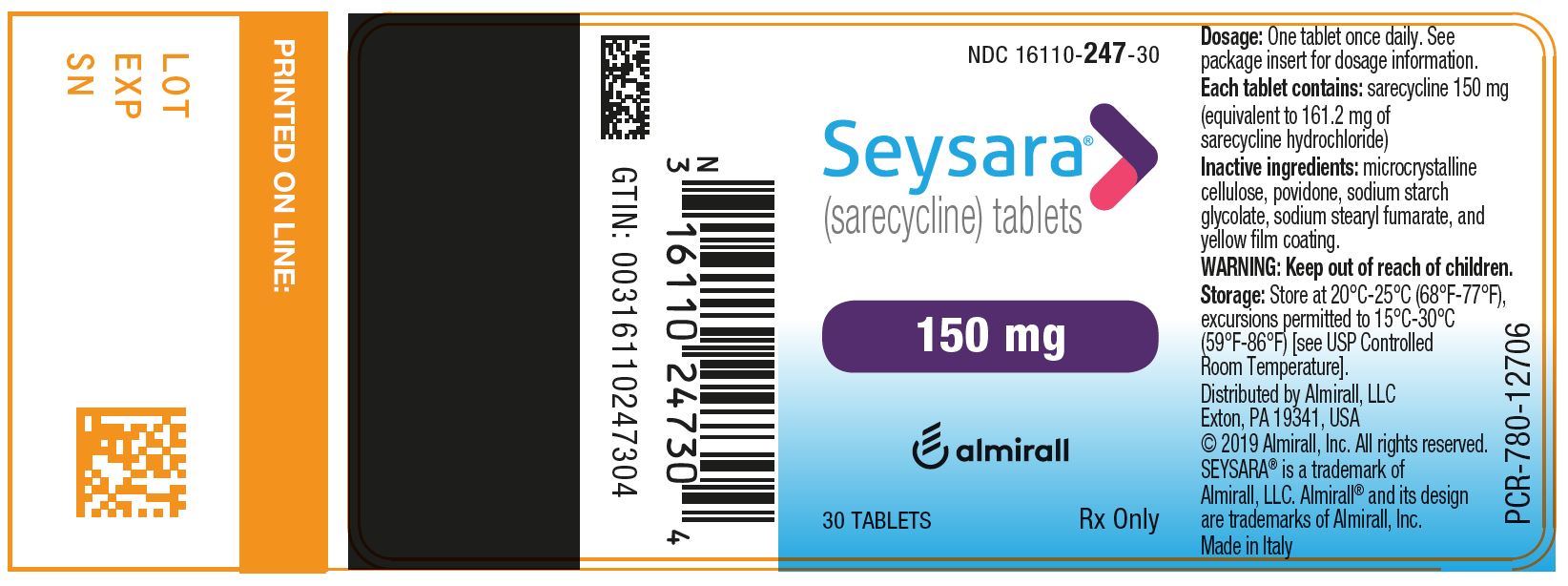
3) SEYSARA (sarecycline) tablets, 150 mg are capsule-shaped, yellow, film-coated tablets debossed with “S150” on one side and blank on the other side.
- Bottles of 30 tablets with child-resistant closure: NDC: 16110-247-30
STORAGE AND HANDLING SECTION
Storage
Store at 20°C - 25°C (68°F - 77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature].
Handling
Protect from moisture and excessive heat.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients taking SEYSARA should receive the following information and instructions:
- SEYSARA should not be used by pregnant women or women attempting to conceive a child [see Use in Specific Populations (8.1)].
- Advise a woman that breastfeeding is not recommended during SEYSARA therapy.
- Advise patients that C. difficile colitis can occur with antibiotic therapy. If patients develop watery or bloody stools, they should seek medical attention.
- Advise patients that intracranial hypertension can occur with tetracycline therapy. If patients experience headache or blurred vision, they should seek medical attention.
- Patients who experience central nervous system symptoms should be cautioned about driving vehicles or using hazardous machinery while on SEYSARA therapy. Patients should seek medical help for persistent central nervous system symptoms.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Advise patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using SEYSARA. If patients need to be outdoors while using SEYSARA, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of skin erythema.
- Advise patients that because of the potential for drug-resistant bacteria to develop during the use of SEYSARA, take SEYSARA as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the current treatment course and increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- Advise patients to drink fluids liberally along with SEYSARA to reduce the risk of esophageal irritation and ulceration [see Dosage and Administration (2)].
© 2019 Almirall, LLC. All rights reserved. SEYSARA® is a registered trademark of Almirall, LLC. Almirall® and its design are trademarks of Almirall, LLC. Distributed by: Almirall, LLC Malvern, PA 19355, USA Almirall
Spl Patient Package Insert Section
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 07/2019
PATIENT INFORMATION
SEYSARA® (SAY' sara) (sarecycline) tablets
What is SEYSARA?
- SEYSARA is a prescription medicine used to treat moderate to severe acne vulgaris in people 9 years and older.
- SEYSARA should not be used for the treatment or prevention of infections
It is not known if SEYSARA is safe and effective for use for longer than 12 weeks. It is not known if SEYSARA is safe and effective in children under 9 years of age.
Do not take SEYSARA:
- if you are allergic to medicines in the tetracycline-class. Ask your healthcare provider or pharmacist for a ul of these medicines if you are not sure.
What should I tell my healthcare provider before taking SEYSARA?
Before taking SEYSARA, tell your healthcare provider about all of your medical conditions, including if you:
- have diarrhea or watery stools
- have vision problems
- are pregnant or plan to become pregnant. SEYSARA may harm your unborn baby. Taking SEYSARA during the second and third trimesters of pregnancy may cause serious side effects on the growth of bone and teeth of your baby. Stop taking SEYSARA and call your healthcare provider right away if you become pregnant during your treatment with SEYSARA.
- are breastfeeding or plan to breastfeed. SEYSARA can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take SEYSARA. You should not breastfeed during treatment with SEYSARA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. SEYSARA and other medicines can affect each other causing serious side effects.
Especially tell your healthcare provider if you take:
- a blood thinner
- a penicillin antibiotic medicine
- antacids that contain aluminum, calcium or magnesium or iron-containing products
- an acne medication taken by mouth that contains isotretinoin or acitretin
How should I take SEYSARA?
- Take SEYSARA exactly as your healthcare provider tells you.
- Skipping doses or not taking all doses of SEYSARA may:
- make the treatment not work as well.
- increase the chance that the bacteria will become resistant to SEYSARA.
- Take SEYSARA 1 time a day with or without food.
- Take SEYSARA with enough fluid to completely swallow the tablet and to lower your risk of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
- If you take too much SEYSARA, stop taking SEYSARA and call your healthcare provider right away or go to the nearest hospital emergency room.
What should I avoid during treatment with SEYSARA?
- Avoid sunlight or artificial sunlight, such as a tanning booth or sunlamp. You could get severe sunburn. Use sunscreen and wear loose-fitting clothes that cover your skin while out in sunlight. Stop taking SEYSARA if you get sunburn.
- You should not drive or operate dangerous machinery until you know how SEYSARA affects you. Tetracyclines may cause you to feel dizzy or lightheaded, or have a spinning feeling (vertigo).
What are the possible side effects of SEYSARA?
SEYSARA may cause serious side effects, including:
- Harm to an unborn baby. See “What should I tell my healthcare provider before taking SEYSARA?”
- Permanent teeth discoloration. SEYSARA may permanently turn a baby or child's teeth yellow-gray-brown during tooth development. You should not use SEYSARA during tooth development. Tooth development happens in the second and third trimesters of pregnancy, and from birth to 8 years of age.
- Slow bone growth. SEYSARA may slow bone growth in infants and children. Slow bone growth is reversible after stopping treatment with SEYSARA.
- Diarrhea. Diarrhea can happen with most antibiotics, including SEYSARA. This diarrhea may be caused by an infection (Clostridium difficile) in your intestines. Call your healthcare provider right away if you get watery or bloody stools.
- Central nervous system effects. See “What should I avoid while taking SEYSARA?” Central nervous system effects such as light headedness, dizziness, and a spinning feeling (vertigo) may go away during your treatment with SEYSARA or if treatment is stopped. Call your healthcare provider if these symptoms do not go away.
- Increased pressure around the brain (intracranial hypertension). This condition may lead to vision changes and permanent vision loss. You may be more likely to get intracranial hypertension if you are a female of childbearing potential and are overweight or have a history of intracranial hypertension. Stop taking SEYSARA and tell your healthcare provider right away if you have blurred vision, vision loss, or headaches.
- Sensitivity to sunlight (photosensitivity). See “What should I avoid during treatment with SEYSARA?”
The most common side effect of SEYSARA is nausea.
SEYSARA may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of SEYSARA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Almirall at 1-866-665-2782.
How should I store SEYSARA?
- Store SEYSARA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SEYSARA away from moisture and excessive heat.
Keep SEYSARA and all medicines out of the reach of children.
General information about the safe and effective use of SEYSARA.
Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use SEYSARA for a condition for which it was not prescribed. Do not give SEYSARA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about SEYSARA that is written for health professionals.
What are the ingredients in SEYSARA?
Active ingredient: sarecycline hydrochloride
Inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, and sodium stearyl fumarate. The yellow film coating contains D&C yellow #10 aluminum lake, iron oxide yellow, methacrylic acid copolymer type C, polyethylene glycol, polyvinyl alcohol, sodium bicarbonate, talc, and titanium dioxide.
© 2019 Almirall, LLC. All rights reserved.                                                                            ALMIRALL
 ALMIRALL
SEYSARA® is a registered trademark of Almirall, LLC.
Almirall® and its design are trademarks of Almirall, LLC.
Distributed by Almirall, LLC
Malvern, PA 19355, USA
Principal Display Panel - Ndc: 16110-245-30 - 60 Mg 30-count Bottle Label
Principal Display Panel - Ndc: 16110-246-30 - 100 Mg 30-count Bottle Label
Principal Display Panel - Ndc: 16110-247-30 - 150 Mg 30-count Bottle Label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site