Sodium Sulfacetamide 9.8% and Sulfur 4.8% Cleanser Dailymed
Generic: sulfacetamide sodium, sulfur is used for the treatment of Corneal Ulcer Ear Diseases Infant, Newborn Dermatitis, Seborrheic Trachoma Skin Diseases, Bacterial Acne Vulgaris Psoriasis Rosacea
Go PRO for all pill images
Rx Only
Description
Each gram of Sodium Sulfacetamide 9.8% - Sulfur 4.8% Cleanser contains 98 mg of Sodium Sulfacetamide and 48 mg of Sulfur in a formulation containing aloe vera gel,butylated hydroxytoluene, cetyl alcohol, disodium edetate, glyceryl monostearate, magnesium aluminum silicate, methylparaben, PEG-40 stearate, PEG -6 caprylic/capric glyceride,PEG-60 almond glyceride, propylparaben, propylene glycol,purified water, sodium thiosulfate, sodium lauryl sulfate, stearyl alcohol, and xanthan gum
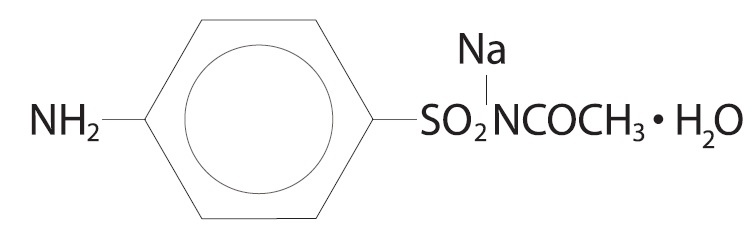
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent.Chemically sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate.The structural formula is:
Clinical Pharmacology
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours.The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
Indications
SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER is indicated for use in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
Contraindications:
SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this preparation. SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER is not to be used by patients with kidney disease.
Warnings
Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome. KEEP OUT OF REACH OF CHILDREN.
Precautions
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.General: If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term studies in animals have not been performed to evaluate carcinogenic potential.Pregnancy: Category C. Animal reproduction studies have not been conducted with SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER. It is also not known whether SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER should be given to a pregnant woman only if clearly needed.Nursing Mothers: It is not known whether sodium sulfacetamide is excreted in human milk following topicaI use of SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER. However, small amounts of orally administered sulfonamides have been reported to be excreted in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER is administered to a nursing woman.Pediatric Use: Safety and effectiveness in children under the age of 12 has not been established.
Adverse Reactions
Although rare, sodium sulfacetamide may cause local irritation. Call your doctor for medical advice about side effects.
Dosage And Administration
Wash affected areas with SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER 1 to 2 times daily or as directed by a physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10 to 20 seconds working into a full lather, rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing cleanser off sooner or using cleanser less often.
How Supplied
SODIUM SULFACETAMIDE 9.8% - SULFUR 4.8% CLEANSER is supplied in an 10oz. (285 g) bottle, NDC 71399-0486-1
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP “Controlled Room Temperature.”]
Note: Protect from freezing and excessive heat. The product may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Keep container or packet tightly closed.
Occasionally, a slight yellowish discoloration may occur when an excessive amount of the product is used and comes in contact with white fabrics. This discoloration, however, presents no problem, as it is readily removed by ordinary laundering without bleaches.All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please NOTE: This is not an Orange Book product and has not been subjected to FDA therapeutic or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendation based on his/her professional knowledge and opinion, upon evaluating the active ingredients, inactive ingredients, excipients and chemical information provided herein.
QUESTIONS? Please Call 1(877) 225-6999
Manufactured for:
Akron Pharma, Inc.
Fairfield, NJ 07004
Manufactured in U.S.A
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


