Sodium Sulfacetamide and Sulfur Dailymed
Generic: sodium sulfacetamide and sulfur is used for the treatment of Corneal Ulcer Ear Diseases Infant, Newborn Dermatitis, Seborrheic Trachoma Skin Diseases, Bacterial Acne Vulgaris Psoriasis Rosacea
Go PRO for all pill images
In a vehicle containing Green Tea and Aloe
Rx Only
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
Description
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate.
The structural formula is:
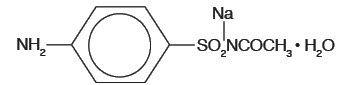
Each mL of Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension contains 80 mg of Sodium Sulfacetamide and 40 mg of Sulfur in a formulation containing Aloe Vera leaf Extract, Butylated Hydroxytoluene, Cetyl Alcohol, Citric Acid, Cocamidopropyl Betaine, Disodium EDTA, Glycerin, Glyceryl Stearate SE, Green Tea Extract, PEG-100 Stearate, Phenoxyethanol, Purified Water, Sodium laureth sulfate, Sodium Thiosulfate, Stearyl alcohol, Triacetin, Xanthan Gum.
Clinical Pharmacology
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours. The exact mode of action of sulfur in the treatment of acne is not known, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
Indications
Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension is indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
Contraindications
This product is contraindicated for use by persons with known or suspected hypersensitivity to sulfonamides, sulfur or any other component of this preparation. This product is not to be used by patients with kidney disease.
Warnings
Although rare, sensitivity to sodium sulfacetaminde may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
FOR EXTERNAL USE ONLY. Keep away from eyes. Keep out of reach of children. Keep container tightly closed.
Precautions
General
If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during longterm therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibilty.
Information for patients
Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
PREGNANCY
Category C
Animal reproduction studies have not been conducted with Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension. It is not known whether Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension. However, small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension is administered to a nursing woman.
PEDIATRIC USE
Safety and effectiveness in children under the age of 12 have not been established.
Adverse Reactions
Although rare, sodium sulfacetamide may cause local irritation.
Call your doctor for medical advice about side effects. To report a serious adverse event, please contact Westminster Pharmaceuticals at 1-844-221-7294.
Overdosage
The oral LD50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately.
Manifestations
Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center (1-800-222-1222), or your doctor.
Dosage And Administration
Apply Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension once or twice daily to affected areas, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed. Massage gently into skin for 10-20 seconds, working into a full lather, rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing off Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension sooner or using less often.
How Supplied
Sodium Sulfacetamide 8% and Sulfur 4% Topical Suspension is available in 16 fl oz (473 mL) bottles, NDC 69367-245-16.
STORAGE AND HANDLING SECTION
Store at controlled room temperature, 15° - 30°C (59° - 86°F). Protect from freezing.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
All prescription substitutions using this product shall be made subject to state and federal statutes as applicable. NOTE: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person's professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical formulation information provided herein.
Manufactured for: Westminster Pharmaceuticals LLCNashville TN, 37217Rev. 05/23
Principal Display Panel - 473 Ml Bottle Label
NDC: 69367-245-16Rx Only
Peel Here
SodiumSulfacetamide 8%and Sulfur 4%Topical Suspension
In a vehicle containingGreen Tea and Aloe
SHAKE WELL
16 fl. oz. (473 mL)
WestminsterPharmaceuticals

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

