tobramycin and dexamethasone Dailymed
Generic: tobramycin and dexamethasone is used for the treatment of Bone Diseases, Infectious Central Nervous System Infections Pseudomonas Infections Respiratory Tract Infections Skin Diseases, Infectious Staphylococcal Infections Urinary Tract Infections Eye Infections, Bacterial Sepsis Intraabdominal Infections Addison Disease Asthma Brain Edema Bursitis Colitis, Ulcerative Collagen Diseases Cushing Syndrome Depressive Disorder Dermatitis Herpetiformis Hypercalcemia Iritis Keratitis Leukemia Lupus Erythematosus, Systemic Mycosis Fungoides Nasal Polyps Nausea Pemphigus Rheumatic Diseases Rhinitis, Allergic, Perennial Sarcoidosis Shock, Septic Spondylitis, Ankylosing Thyroiditis Tuberculosis, Ocular Tuberculosis, Pulmonary Vomiting Eye Infections, Fungal Eye Infections, Viral Purpura, Thrombocytopenic, Idiopathic
Go PRO for all pill images
Description
Tobramycin and dexamethasone ophthalmic suspension, USP is a sterile, multiple-dose antibiotic and steroid combination for topical ophthalmic use. The chemical structures for tobramycin and dexamethasone are presented below:
Tobramycin, USP
Tobramycin, USP is a white to off-white, hygroscopic powder. It is freely soluble in water, very slightly soluble in alcohol, practically insoluble in chloroform and in ether.
Chemical Structure:
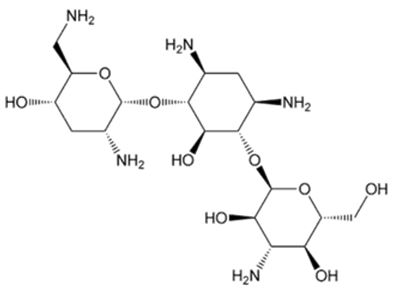
Molecular Formula: C18H37N5O9 Molecular Weight: 467.51Chemical Name: O-3-Amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl (1→6)]-2-deoxy-L-streptamine
Dexamethasone, USP
Dexamethasone, USP (Micronised) is a white or practically white crystalline powder.
Chemical Structure:
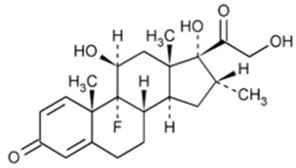
Molecular Formula: C22H29FO5 Molecular Weight: 392.46Chemical Name: 9-Fluoro-11ß,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione
Each mL of tobramycin and dexamethasone ophthalmic suspension, USP contains:
Active: tobramycin, USP 0.3% (3 mg) and dexamethasone, USP 0.1% (1 mg).
Preservative: benzalkonium chloride 0.01%.
Inactives: tyloxapol, edetate disodium, sodium chloride, hydroxyethyl cellulose, sodium sulfate, sulfuric acid and/or sodium hydroxide (to adjust pH) and  water for injection, USP.
Clinical Pharmacology
Corticoids suppress the inflammatory response to a variety of agents and they probably delay or slow healing. Since corticoids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant. Dexamethasone is a potent corticoid.
The antibiotic component in the combination (tobramycin) is included to provide action against susceptible organisms. In vitro studies have demonstrated that tobramycin is active against susceptible strains of the following microorganisms:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
No data are available on the extent of systemic absorption from tobramycin and dexamethasone ophthalmic suspension; however, it is known that some systemic absorption can occur with ocularly applied drugs.
Indications And Usage
Tobramycin and dexamethasone ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drug in this product is active against the following common bacterial eye pathogens:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
Contraindications
Epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and many other viral diseases of the cornea and conjunctiva. Mycobacterial infection of the eye. Fungal diseases of ocular structures. Hypersensitivity to a component of the medication.
Warnings
FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION INTO THE EYE.
Sensitivity to topically applied aminoglycosides may occur in some patients. Severity of hypersensitivity reactions may vary from local effects to generalized reactions, such as erythema, itching, urticaria, skin rash, anaphylaxis, anaphylactoid reactions, or bullous reactions. If a sensitivity reaction does occur, discontinue use.
Prolonged use of steroids may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Intraocular pressure (IOP) should be routinely monitored even though it may be difficult in pediatric patients and uncooperative patients. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions and parasitic infections of the eye, steroids may mask infection or enhance existing infection.
In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids.
Precautions
General
The possibility of fungal infections of the cornea should be considered after long-term steroid dosing. As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated. When multiple prescriptions are required, or whenever clinical judgement dictates, the patient should be examined with the aid of magnification, such as slit lamp biomicroscopy and, where appropriate, fluorescein staining.
Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the contents. Contact lenses should not be worn during the use of this product.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to evaluate the carcinogenic or mutagenic potential. No impairment of fertility was noted in studies of subcutaneous tobramycin in rats at doses of 50 and 100 mg/kg/day.
Pregnancy
Corticosteroids have been found to be teratogenic in animal studies. Ocular administration of 0.1% dexamethasone resulted in 15.6% and 32.3% incidence of fetal anomalies in two groups of pregnant rabbits. Fetal growth retardation and increased mortality rates have been observed in rats with chronic dexamethasone therapy. Reproduction studies have been performed in rats and rabbits with tobramycin at doses up to 100 mg/kg/day parenterally and have revealed no evidence of impaired fertility or harm to the fetus.
There are no adequate and well-controlled studies in pregnant women. However, prolonged or repeated corticoid use during pregnancy has been associated with an increased risk of intra-uterine growth retardation. Tobramycin and dexamethasone ophthalmic suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be observed carefully for signs of hypoadrenalism.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when tobramycin and dexamethasone ophthalmic suspension is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Adverse Reactions
Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination. Exact incidence figures are not available.
The most frequent adverse reactions to topical ocular tobramycin (tobramycin ophthalmic solution, 0.3%) are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than 4% of patients. The reactions due to the steroid component are: elevation of IOP with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
Secondary Infection
The development of secondary infection has occurred after use of combinations containing steroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion must be considered in any persistent corneal ulceration where steroid treatment has been used. Secondary bacterial ocular infection following suppression of host responses also occurs.
Postmarketing Experience
Additional adverse reactions identified from postmarketing use include anaphylactic reaction, erythema multiforme.
The following additional adverse reactions have been reported with the individual components uled below:
Dexamethasone: Cushing’s syndrome and adrenal suppression may occur after use of dexamethasone in excess of the uled dosing instructions in predisposed patients, including children and patients treated with CYP3A4 inhibitors.
Aminoglycosides: Neurotoxicity, ototoxicity, and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, because of their potential effect on neuromuscular function.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dosage And Administration
One or two drops instilled into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to one or two drops every two (2) hours. Frequency should be decreased gradually as warranted by improvement in clinical signs. Care should be taken not to discontinue therapy prematurely.
Not more than 20 mL should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above.
How Supplied
Tobramycin and Dexamethasone Ophthalmic Suspension USP, 0.3% and 0.1% is a sterile, white to off-white uniform suspension, essentially free from foreign matter, supplied in 2.5 mLÂ 5 mL or 10 mL Â LDPE natural bottle with LDPE natural nozzle and HDPE white cap. Each mL contains tobramycin, USP 0.3% (3 mg) and dexamethasone, USP 0.1% (1 mg).
It is available as follows:
2.5 mL in 5 mL Container:Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â NDC 69238-1373-2
5 mL in 5 mL Container:Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â NDC 69238-1374-3
10 mL in 10 mL Container:Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â NDC 69238-1375-6
Storage
Store at 8º to 27ºC (46º to 80ºF).
Store suspension upright and shake well before using.
After opening, tobramycin and dexamethasone ophthalmic suspension can be used until the expiration date on the bottle.
Manufactured by: Amneal Pharmaceuticals Pvt. Ltd.Parenteral Unit Ahmedabad 382213, INDIA
Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807
Rev. 05-2021-05
Principal Display Panel
NDC 69238-1373-2Tobramycin and Dexamethasone Ophthalmic Solution USP, 0.3%/0.1%Rx Only2.5 mLAmneal Pharmaceuticals LLC

Â

NDC 69238-1374-3Tobramycin and Dexamethasone Ophthalmic Solution USP, 0.3%/0.1%Rx Only5 mLAmneal Pharmaceuticals LLC
Â

Â
NDC 69238-1375-6Tobramycin and Dexamethasone Ophthalmic Solution USP, 0.3%/0.1%Rx Only10 mLAmneal Pharmaceuticals LLC


Â
Â
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


