SOAANZ Dailymed
Generic: torsemide is used for the treatment of Anuria Ascites Dehydration Edema Heart Failure Hypertension Kidney Failure, Chronic Liver Cirrhosis Nephrotic Syndrome Pulmonary Edema Acute Kidney Injury
Go PRO for all pill images
1 Indications And Usage
SOAANZ is indicated in adults for the treatment of edema associated with heart failure or renal disease.
SOAANZ is a loop diuretic indicated in adults for the treatment of edema associated with heart failure or renal disease. (1 )
2 Dosage And Administration
The recommended initial dose is 20 mg oral SOAANZ once daily. If the diuretic response is inadequate, titrate upward by approximately doubling until the desired diuretic response is obtained. Doses higher than 200 mg have not been adequately studied.
The recommended initial dose is 20 mg orally once daily. Titrate dose by approximately doubling until desired diuretic response is obtained. Doses above 200 mg have not been studied.(2 )
3 Dosage Forms And Strengths
Tablets: 20 mg, 40 mg and 60 mg (3 )
Tablets:
- 20 mg, yellow, round, film-coated tablet debossed with "T20" on one side
- 40 mg, orange, round, film-coated tablet debossed with "T40" on one side
- 60 mg, pink, round, film-coated tablet debossed with "T60" on one side
4 Contraindications
SOAANZ is contraindicated in patients with known hypersensitivity to SOAANZ.
SOAANZ is contraindicated in patients who are anuric.
SOAANZ is contraindicated in patients with hepatic coma.
Hypersensitivity to SOAANZ, anuria, and hepatic coma. (4 )
5 Warnings And Precautions
- Hypotension and worsening renal function: monitor volume status and renal function periodically (
5.1 )- Electrolyte and metabolic abnormalities: monitor serum electrolytes and blood glucose periodically. (
5.2 )- Ototoxicity (
5.3 ,7.6 )5.1 Hypotension and Worsening Renal Function
Excessive diuresis may cause potentially symptomatic dehydration, blood volume reduction and hypotension and worsening renal function, including acute renal failure particularly in salt-depleted patients or those taking renin-angiotensin aldosterone inhibitors. Worsening of renal function can also occur with concomitant use of nephrotoxic drugs. Monitor volume status and renal function periodically.
5.2 Electrolyte and Metabolic Abnormalities
SOAANZ can cause potentially symptomatic hypokalemia, hyponatremia, hypomagnesemia, hypocalcemia, and hypochloremic alkalosis. Treatment with SOAANZ can cause an increase in blood glucose levels and hyperglycemia. Asymptomatic hyperuricemia can occur, and gout may rarely be precipitated. Monitor serum electrolytes and blood glucose periodically.
5.3 Ototoxicity
Tinnitus and hearing loss (usually reversible) have been observed with loop diuretics, including torsemide. Higher than recommended doses, severe renal impairment, and hypoproteinemia, appear to increase the risk of ototoxicity.
6 Adverse Reactions
The following risks are discussed in more detail in other sections:
- Hypotension and Worsening Renal Function [see Warnings and Precautions (5.1)]
- Electrolyte and Metabolic Abnormalities [see Warnings and Precautions (5.2)]
- Ototoxicity [see Warnings and Precautions (5.3)]
Discontinuation of therapy due to adverse reactions occurred in 6% of patients treated with SOAANZ (6.1 ).
To report SUSPECTED ADVERSE REACTIONS, contact Sarfez Pharmaceuticals Inc. at 1-703-627-1934 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In pre-approval studies, SOAANZ has been evaluated for safety in 65 subjects. Discontinuation of therapy due to adverse reactions occurred in 4 out of the 65 of subjects (6%) treated with SOAANZ.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the post-approval use of torsemide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
Gastrointestinal System: Pancreatitis, abdominal pain
Nervous System: Paresthesia, confusion, visual impairment, loss of appetite
Hematologic: Leucopenia, thrombocytopenia, anemia
Hepatobiliary: Increase in liver transaminases, gamma-glutamyltransferase
Metabolism: Thiamine (vitamin B1) deficiency
Skin/hypersensitivity: Stevens-Johnson syndrome, toxic epidermal necrolysis, photosensitivity reaction, pruritus
Urogenital: Acute urinary retention
7 Drug Interactions
- Non-steroidal anti-inflammatory drugs (NSAIDs): Reduced diuretic, natriuretic, and antihypertensive effects; risk of renal impairment. (
7.1 )- CYP2C9: Concomitant use with CYP2C9 inhibitors can decrease torsemide clearance. Torsemide may affect the efficacy and safety of sensitive CYP2C9 substrates or of substrates with a narrow therapeutic range, such as warfarin or phenytoin. (
7.2 )- Cholestyramine: Decreased exposure of SOAANZ. (
7.3 )- Organic anion drugs: may decrease diuretic activity of SOAANZ. (
7.4 )- Lithium: Risk of lithium toxicity. (
7.5 )- Renin-angiotensin inhibitors: Increased risk of hypotension and renal impairment. (
7.7 )- Radiocontrast agents: Increased risk of renal toxicity. (
7.8 )- Corticosteroids and ACTH: Increased risk of hypokalemia. (
7.9 )7.1 Nonsteroidal Anti-inflammatory Drugs
Because torsemide and salicylates compete for secretion by renal tubules, patients receiving high doses of salicylates may experience salicylate toxicity when SOAANZ is concomitantly administered.
Concomitant use of nonsteroidal anti-inflammatory drugs (NSAIDs) and torsemide has been associated with the development of acute renal failure. The diuretic effects of torsemide can be reduced by NSAIDs.
Partial inhibition of the natriuretic effect of torsemide by concomitant administration of indomethacin has been demonstrated for torsemide under conditions of dietary sodium restriction (50 mEq/day) but not in the presence of normal sodium intake (150 mEq/day).
7.2 Cytochrome P450 2C9 Inhibitors and Inducers
Torsemide is a substrate of CYP2C9. Concomitant use of CYP2C9 inhibitors (e.g., amiodarone, fluconazole, miconazole, oxandrolone) can decrease torsemide clearance and increase torsemide plasma concentrations. Concomitant use of CYP2C9 inducers (e.g., rifampin) increase torsemide clearance and decrease plasma torsemide concentrations. Monitor diuretic effect and blood pressure when used in combination with CYP2C9 inhibitor or inducer. Adjust SOAANZ dose, if necessary.
Because of its inhibition of CYP2C9 metabolism, torsemide may affect the efficacy and safety of sensitive CYP2C9 substrates, such as celecoxib, or of substrates with a narrow therapeutic range, such as warfarin or phenytoin. Monitor patients and adjust dosages if necessary.
7.3 Cholestyramine
Concomitant use of torsemide and cholestyramine has not been studied in humans but, in a study in animals, coadministration of cholestyramine decreased the absorption of orally administered torsemide.
If SOAANZ and cholestyramine should be coadministered, administer SOAANZ at least one hour before or 4 to 6 h after cholestyramine administration.
7.4 Organic Anion Drugs
Coadministration of organic anion drugs (e.g., probenecid) that undergo significant renal tubular secretion have the potential to reduce secretion of torsemide into the proximal tubule which thereby decreases the diuretic activity of SOAANZ. Monitor diuretic effect and blood pressure during coadministration.
7.5 Lithium
Like other diuretics, torsemide reduces the renal clearance of lithium, inducing a high risk of lithium toxicity. Monitor lithium levels periodically when SOAANZ is coadministered.
7.6 Ototoxic Drugs
Loop diuretics increase the ototoxic potential of other ototoxic drugs, including aminoglycoside antibiotics and ethacrynic acid. This effect has been reported with concomitant use of torsemide and gentamycin. Avoid concomitant use of SOAANZ and aminoglycoside antibiotics, if possible.
7.7 Renin-angiotensin Inhibitors
Coadministration of SOAANZ with ACE inhibitors or angiotensin receptor blockers can increase the risk of hypotension and renal impairment.
7.8 Radiocontrast Agents
SOAANZ can increase the risk of renal toxicity related to administration of radiocontrast agents.
7.9 Corticosteroids and ACTH
Concomitant use with SOAANZ may increase risk of hypokalemia.
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on use of SOAANZ in pregnant women and the risk of major birth defects or miscarriage. In pregnant rats and rabbits dosed, on a mg/m2 basis, with 2.4 and 1.6 times a human starting dose of 20 mg/day, respectively, there was no fetotoxicity or teratogenicity. However, in pregnant rats and rabbits administered 50 and 6.8 times the human dose, respectively, decreases in body weight, decreased fetal resorption and delayed fetal ossification was observed.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major malformations and miscarriage in clinically recognized pregnancies is 2-4%, and 15-20%, respectively.
Animal Data
There was no fetotoxicity or teratogenicity in rats treated with up to 5 mg/kg/day of torsemide (on a mg/m2 basis, the animal dose is 2.4 times the human starting dose of 20 mg/day), or in rabbits, treated with 1.6 mg/kg/day (on a mg/m2 basis, the animal dose is 1.6 times the human starting dose of 20 mg/day dose). Fetal and maternal toxicity (decrease in average body weight, increase in fetal resorption and delayed fetal ossification) occurred in rabbits and rats given doses 4 (rabbits) and 5 (rats) times larger.
8.2 Lactation
Risk Summary
There are no data regarding the presence of torsemide in human milk or the effects of torsemide on the breastfed child. Diuretics can suppress lactation.
8.4 Pediatric Use
The safety and effectiveness of SOAANZ in pediatric patients have not been established.
Administration of a loop diuretic to premature infants has been associated with the precipitation of nephrocalcinosis/nephrolithiasis. Nephrocalcinosis/nephrolithiasis has also been observed in children under 4 years of age with no history of prematurity who have been treated chronically with a loop diuretic. A loop diuretic, when administered during the first weeks of life, has also been reported to increase the risk of persistent patent ductus arteriosus.
8.5 Geriatric Use
Of the total number of patients who received torsemide in United States clinical studies, 24% were 65 or older and about 4% were 75 or older. No specific age-related differences in effectiveness or safety were observed between younger patients and elderly patients.
8.6 Use in Renal Impairment
In patients with non-anuric renal failure, high doses of torsemide (20 mg to 200 mg) caused marked increases in water and sodium excretion. In patients with non-anuric renal failure, severe enough to require hemodialysis, chronic treatment with up to 200 mg of daily torsemide has not been shown to change steady-state fluid retention. When patients in a study of acute renal failure received total daily doses of 520 mg to 1200 mg of torsemide, 19% experienced seizures. Ninety-six patients were treated in this study; 6/32 treated with torsemide experienced seizures, 6/32 treated with comparably high doses of furosemide experienced seizures, and 1/32 treated with placebo experienced a seizure. [see Dosage and Administration (2.1)].
10 Overdosage
The signs and symptoms of overdosage can be anticipated to include those of excessive pharmacologic effect: dehydration, hypovolemia, hypotension, hyponatremia, hypokalemia, hypochloremic alkalosis, and hemoconcentration. Treatment of overdosage should consist of fluid and electrolyte replacement.
Laboratory determinations of serum levels of torsemide and its metabolites are not widely available.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of torsemide and its metabolites. Torsemide is not dialyzable, so hemodialysis will not accelerate elimination.
11 Description
SOAANZ contains torsemide, a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:

Its empirical formula is C16H20N4O3S, its pKa is 6.42, and its molecular weight is 348.42.
Torsemide is a white to off-white crystalline powder. The tablets for oral administration also contain colloidal silicon dioxide, hypromellose, iron oxide yellow, iron oxide red, lactose, magnesium stearate, microcrystalline cellulose, talc, polyethylene glycol and titanium dioxide.
12 Clinical Pharmacology
12.1 Mechanism of Action
Micropuncture studies in animals have shown that torsemide acts from within the lumen of the thick ascending portion of the loop of Henle, where it inhibits the Na+/K+/2Cl--carrier system. Clinical pharmacology studies have confirmed this site of action in humans, and effects in other segments of the nephron have not been demonstrated. Diuretic activity thus correlates better with the rate of drug excretion in the urine than with the concentration in the blood.
Torsemide increases the urinary excretion of sodium, chloride, and water, but it does not significantly alter glomerular filtration rate, renal plasma flow, or acid-base balance.
12.2 Pharmacodynamics
With oral dosing of SOAANZ, the onset of diuresis occurs within 1 hour and the peak effect occurs during the first four hours and diuresis lasts about 6 to 8 hours.The mean increase in potassium excretion over 23 hours following a single oral dose of SOAANZ 20 mg in healthy adults was 12.7 mEq.
Edema
Torsemide has been studied in controlled trials in patients with New York Heart Association Class II to Class IV heart failure. Patients who received 10 mg to 20 mg of daily torsemide in these studies achieved significantly greater reductions in weight and edema than did patients who received placebo.
When torsemide is first administered, daily urinary sodium excretion increases for at least a week. With chronic administration, however, daily sodium loss comes into balance with dietary sodium intake. If the administration of torsemide is suddenly stopped, blood pressure returns to pretreatment levels over several days, without overshoot.
Torsemide has been administered together with β-adrenergic blocking agents, ACE inhibitors, and calcium-channel blockers. Adverse drug interactions have not been observed, and special dosage adjustment has not been necessary.
12.3 Pharmacokinetics
Absorption
Torsemide reaches peak plasma concentration (Cmax) within 2.5 hours after oral administration of SOAANZ. Cmax and area under the serum concentration-time curve (AUC) of torsemide after oral administration are proportional to dose over the range of 2.5 mg to 200 mg.
Effect of Food
Administration of SOAANZ with a high fat, high calorie meal in healthy adults delays the time to Cmax by about 45 minutes, increases Cmax by 100% and increases area under the plasma concentration over time curve (AUC) by 48%. No clinically significant change in overall diuretic activity was observed.
Distribution
The volume of distribution of torsemide is 12 to 15 liters in normal adults or in patients with mild to moderate renal failure or congestive heart failure. In patients with hepatic cirrhosis, the volume of distribution is approximately doubled. Torsemide is extensively bound to plasma protein (>99%).
Metabolism
Torsemide is metabolized by the hepatic cytochrome CYP2C9 and, to a minor extent, CYP2C8 and CYP2C18. Three main metabolites have been identified in humans. Metabolite M1 is formed by methylhydroxylation of torsemide, metabolite M3 is formed by ring hydroxylation of torsemide, and metabolite M5 is formed by oxidation of M1. The major metabolite in humans is the carboxylic acid derivative M5, which is biologically inactive. Metabolites M1 and M3 possess some pharmacological activity; however, their systemic exposures are much lower when compared to torsemide.
Elimination
In normal subjects the elimination half-life of torsemide is approximately 3.5 hours. Torsemide is cleared from the circulation by both hepatic metabolism (approximately 80% of total clearance) and excretion into the urine (approximately 20% of total clearance in patients with normal renal function).
Because torsemide is extensively bound to plasma protein (>99%), very little enters tubular urine via glomerular filtration. Most renal clearance of torsemide occurs via active secretion of the drug by the proximal tubules into tubular urine.
After a single oral dose, the amounts recovered in urine were: torsemide 21%, metabolite M1 12%, metabolite M3 2%, and metabolite M5 34%.
Renal Impairment
In patients with renal failure, renal clearance of torsemide is markedly decreased but total plasma clearance is not significantly altered. A smaller fraction of the administered dose is delivered to the intraluminal site of action, and the natriuretic action of any given dose of diuretic is reduced.
Hepatic Impairment
In patients with hepatic cirrhosis, the volume of distribution, plasma half-life, and renal clearance are all increased, but total clearance is unchanged.
Geriatric Patients
The renal clearance of torsemide is lower in elderly subjects as compared to younger adults, which is related to the decline in renal function that commonly occurs with aging. However, total plasma clearance and elimination half-life remain unchanged.
Heart Failure
In patients with decompensated congestive heart failure, hepatic and renal clearance are both reduced, probably because of hepatic congestion and decreased renal plasma flow, respectively. The total clearance of torsemide is approximately 50% of that seen in healthy volunteers, and the plasma half-life and AUC are correspondingly increased. Because of reduced renal clearance, a smaller fraction of any given dose is delivered to the intraluminal site of action, so at any given dose there is less natriuresis in patients with heart failure than in normal subjects.
Drug Interactions
Digoxin: Coadministration of digoxin is reported to increase the AUC for torsemide by 50%, but dose adjustment of SOAANZ is not necessary. Torsemide does not affect the pharmacokinetics of digoxin.
Spironolactone: In healthy subjects, coadministration of torsemide was associated with significant reduction in the renal clearance of spironolactone, with corresponding increases in the AUC. However, the pharmacokinetic profile and diuretic activity of torsemide are not altered by spironolactone.
Torsemide does not affect the protein binding of glyburide or warfarin.
Cimetidine: The pharmacokinetic profile and diuretic activity of torsemide are not altered by cimetidine.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No overall increase in tumor incidence was found when torsemide was given to rats and mice throughout their lives at doses up to 9 mg/kg/day (rats) and 32 mg/kg/day (mice). On a body-weight basis, these doses are 27 to 96 times a human dose of 20 mg; on a body-surface-area basis, they are 5 to 8 times this dose. In the rat study, the high-dose female group demonstrated renal tubular injury, interstitial inflammation, and a statistically significant increase in renal adenomas and carcinomas. The tumor incidence in this group was, however, not much higher than the incidence sometimes seen in historical controls. Similar signs of chronic non-neoplastic renal injury have been reported in high-dose animal studies of other diuretics such as furosemide and hydrochlorothiazide.
No mutagenic activity was detected in any of a variety of in vivo and in vitro tests of torsemide and its major human metabolite. The tests included the Ames test in bacteria (with and without metabolic activation), tests for chromosome aberrations and sister-chromatid exchanges in human lymphocytes, tests for various nuclear anomalies in cells found in hamster and murine bone marrow, tests for unscheduled DNA synthesis in mice and rats, and others.
In doses up to 25 mg/kg/day (75 times a human dose of 20 mg on a body-weight basis; 13 times this dose on a body-surface-area basis), torsemide had no adverse effect on the reproductive performance of male or female rats.
16 How Supplied/storage And Handling
SOAANZ tablets are available as round, film-coated, debossed on one side in the following configurations:
Strength Color Debossing Bottle of 30 tablets Bottle of 90 Tablets 20 mg Yellow T20 NDC 73502-786-07 NDC 73502-786-08 40 mg Orange T40 NDC 73502-586-10 NDC 73502-586-11 60 mg Pink T60 NDC 73502-687-13 NDC 73502-687-14
Strength Color Debossing Tablets per Card Carton of 30 Tablets 20 mg Yellow T20 6 NDC 73502-286-09 40 mg Orange T40 6 NDC 73502-288-15 60 mg Pink T60 6 NDC 73502-287-12 STORAGE AND HANDLING SECTION
Store between 20°C and 25°C (68°F and 77°F). Excursions permitted between 15°C and 30°C (59° F and 86°F).
17 Patient Counseling Information
Symptomatic Hypotension: Advise patients receiving SOAANZ that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. Patients should be told that if syncope occurs, SOAANZ should be discontinued until the physician has been consulted.
All patients should be cautioned that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope [see Warnings and Precautions (5.1)].
Non-Steroidal Anti-inflammatory Drugs (NSAID): Advise patients to discuss with their physician before taking NSAID medications concomitantly [see Drug Interactions (7.1)].
SARFEZ and SOAANZ are registered trademarks of Sarfez. Any other trademarks are the property of their respective owners.
Manufactured For:Sarfez Pharmaceuticals Inc. Vienna, VA 22182
© 2021 Sarfez Pharmaceuticals Inc.
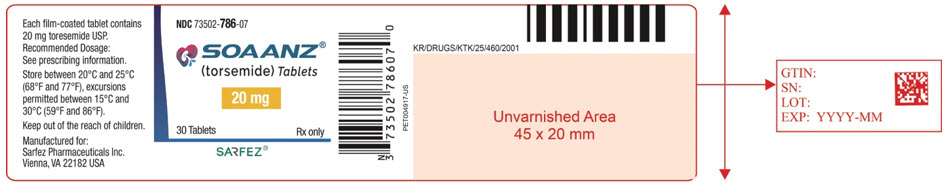
Principal Display Panel - 20 Mg Tablet Bottle Label - 786-07
NDC 73502-786-07
SOAANZ® (torsemide) Tablets
20 mg
30 Tablets Rx only
SARFEZ®
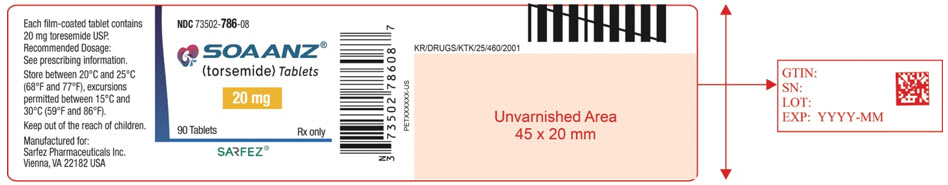
Principal Display Panel - 20 Mg Tablet Bottle Label - 786-08
NDC 73502-786-08
SOAANZ® (torsemide) Tablets
20 mg
90 Tablets Rx only
SARFEZ®
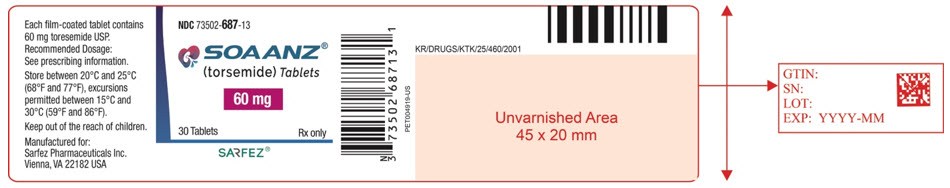
Principal Display Panel - 60 Mg Tablet Bottle Label - 687-13
NDC 73502-687-13
SOAANZ® (torsemide) Tablets
60 mg
30 Tablets Rx only
SARFEZ®
Principal Display Panel - 60 Mg Tablet Bottle Label - 687-14
NDC 73502-687-14
SOAANZ® (torsemide) Tablets
60 mg
90 Tablets Rx only
SARFEZ®

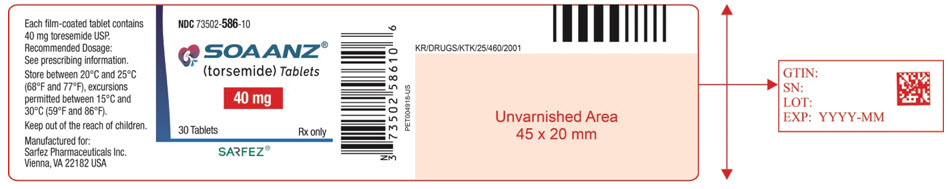
Principal Display Panel - 40 Mg Tablet Bottle Label - 586-10
NDC 73502-586-10
SOAANZ® (torsemide) Tablets
40 mg
30 Tablets Rx only
SARFEZ®
Principal Display Panel - 40 Mg Tablet Bottle Label - 586-11
NDC 73502-586-11
SOAANZ® (torsemide) Tablets
40 mg
90 Tablets Rx only
SARFEZ®
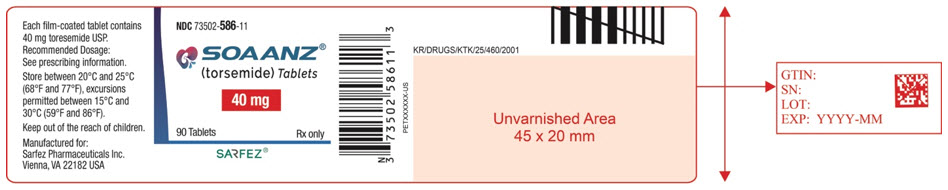
Principal Display Panel - 20 Mg Tablet Blister Pack Label
NDC 73502-286-09
SOAANZ® (torsemide) Tablets
20 mg
Manufactured for: Sarfez Pharmaceuticals Inc.
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:

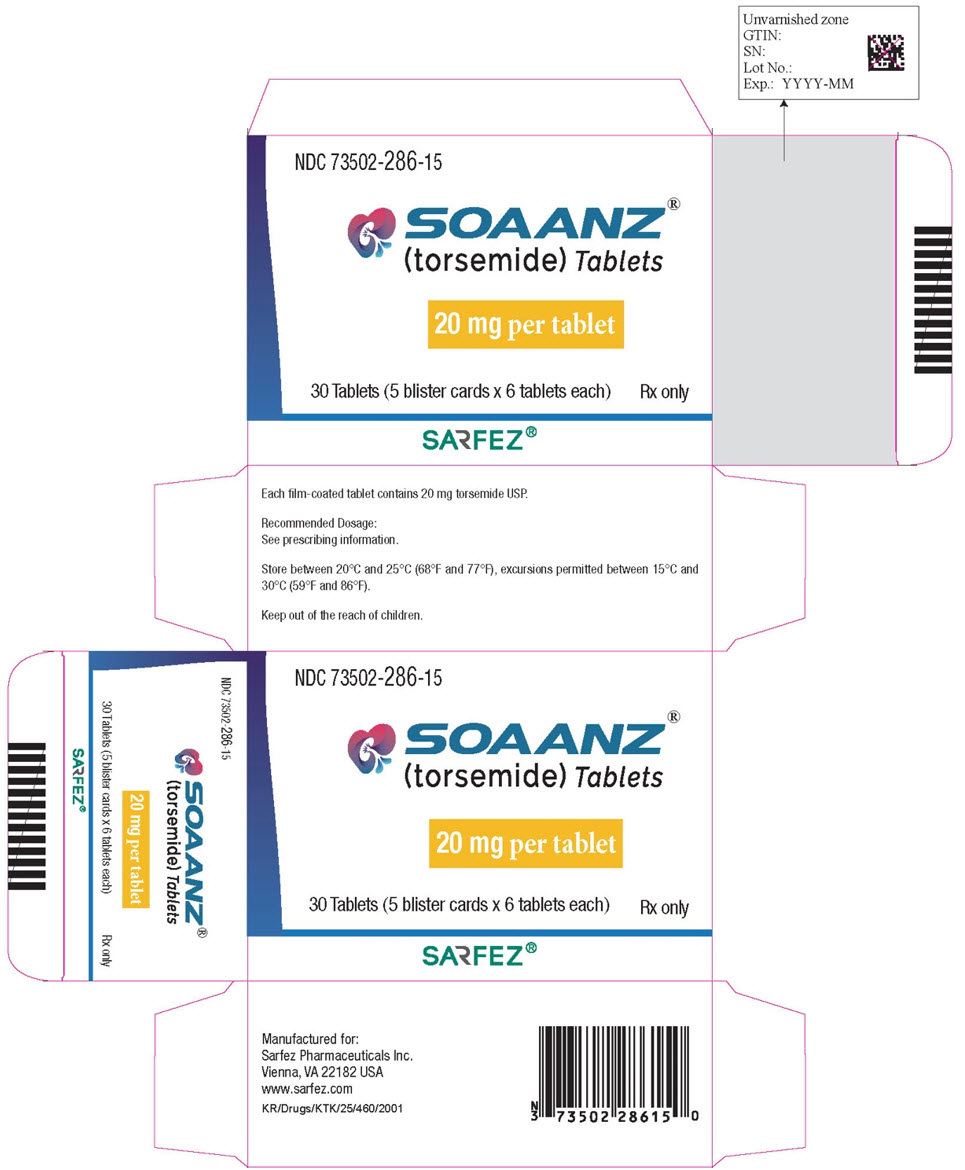
Principal Display Panel - 20 Mg Tablet Blister Pack Carton
NDC 73502-286-15
SOAANZ® (torsemide) Tablets
20 mg per tablet
30 Tablets (5 buler cards x 6 tablets each)
Rx only
SARFEZ®
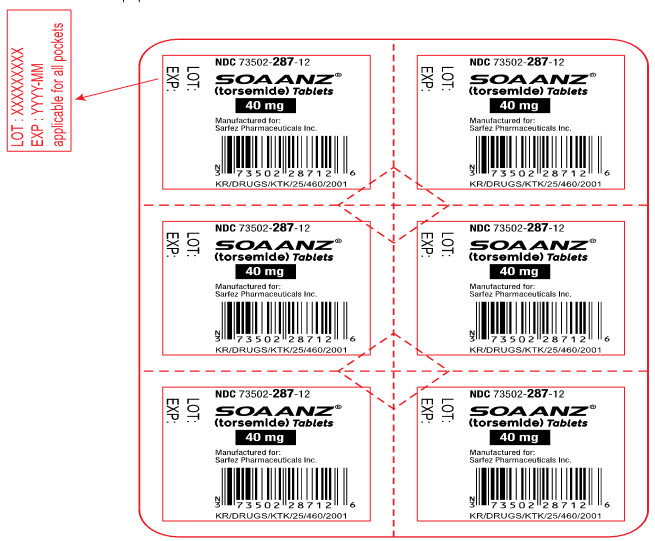
Principal Display Panel - 40 Mg Tablet Blister Pack Label
NDC 73502-287-12
SOAANZ® (torsemide) Tablets
40 mg
Manufactured for: Sarfez Pharmaceuticals Inc.
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:
Principal Display Panel - 40 Mg Tablet Blister Pack Carton
NDC 73502-287-16
SOAANZ® (torsemide) Tablets
40 mg per tablet
30 Tablets (5 buler cards x 6 tablets each)
Rx only
SARFEZ®

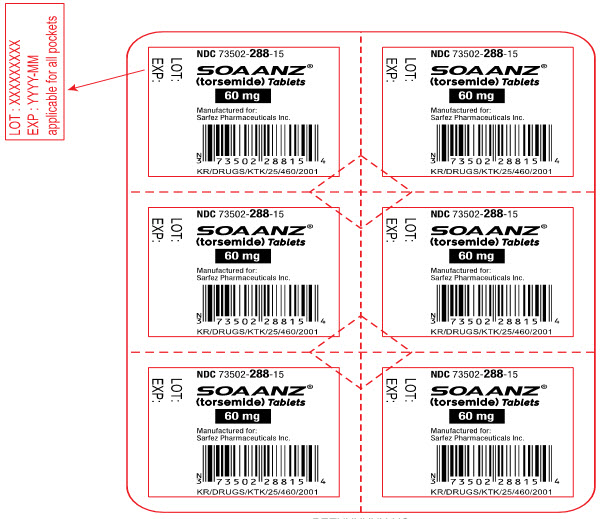
Principal Display Panel - 60 Mg Tablet Blister Pack Label
NDC 73502-288-15
SOAANZ® (torsemide) Tablets
60 mg
Manufactured for: Sarfez Pharmaceuticals Inc.
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:
Principal Display Panel - 60 Mg Tablet Blister Pack Carton
NDC 73502-288-17
SOAANZ® (torsemide) Tablets
60 mg per tablet
30 Tablets (5 buler cards x 6 tablets each)
Rx only
SARFEZ®

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


