Urinary Pain Relief (urinary pain relief (phenazopyridine hydrochloride) tablet) Dailymed
Generic: phenazopyridine hydrochloride is used for the treatment of Kidney Diseases Liver Diseases Pain Pyelonephritis Urological Manifestations
IMPRINT: S159
SHAPE: round
COLOR: brown
All Imprints
urinary pain relief (phenazopyridine hydrochloride) tablet - s 159 round brown
urinary pain relief (phenazopyridine hydrochloride) tablet - s159 round brown
Go PRO for all pill images
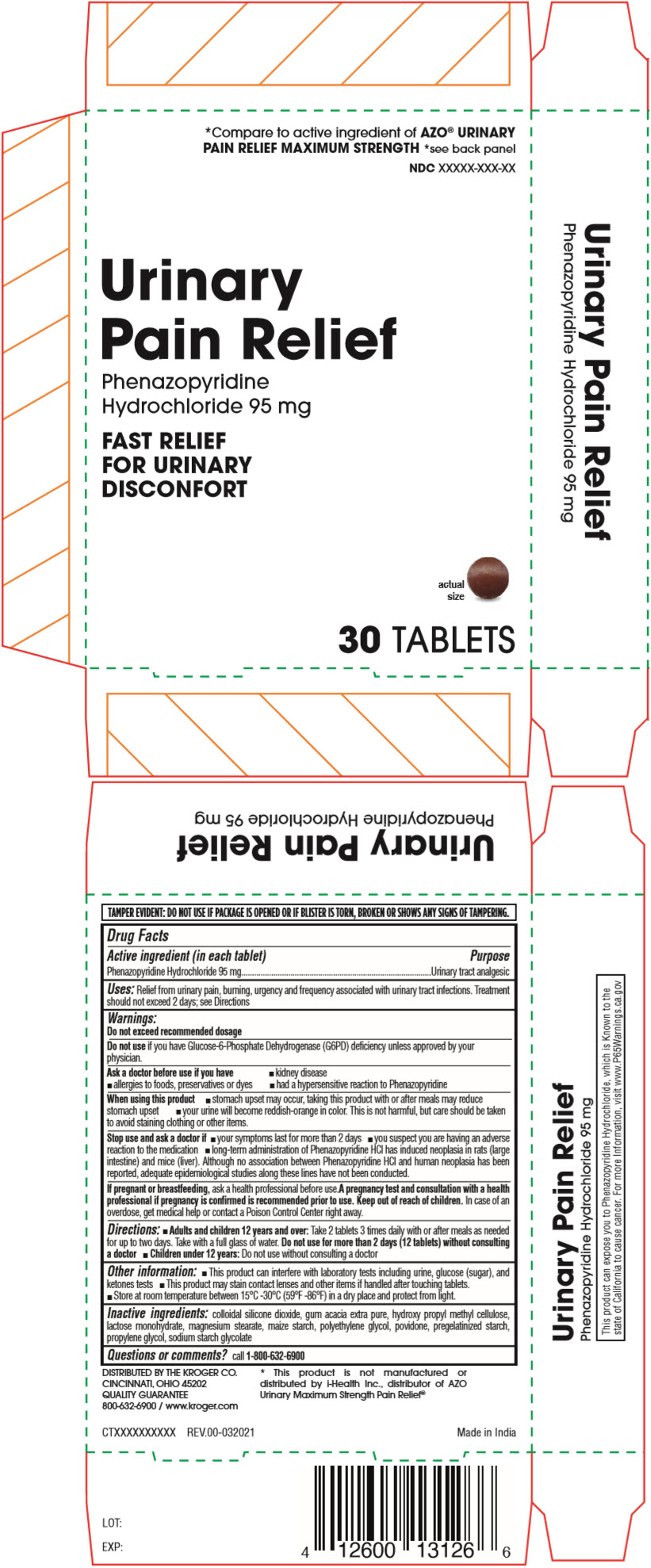
Drug Facts
Active Ingredient (in Each Tablet)
Phenazopyridine Hydrochloride 95 mg
Purpose
Urinary tract analgesic
Uses
Relief from urinary pain, burning, urgency and frequency associated with urinary tract infections. Treatment should not exceed 2 days; see Directions
Warnings
Do not exceed recommended dosage
OTC - DO NOT USE SECTION
Do not use if you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine
When using this product
- stomach upset may occur, taking this product with or after meals may reduce stomach upset
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other lis.
Stop use and ask a doctor if
- your symptoms last for more than 2 days
- you suspect you are having an adverse reaction to the medication
- long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver). Although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breastfeeding, ask a health professional before use.A pregnancy test and consultation with a health professional if pregnancy is confirmed is recommended prior to use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of an overdose, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years and over: Take 2 tablets 3 times daily with or after meals as needed for up to two days. Take with a full glass of water. Do not use for more than 2 days (12 tablets) without consulting a doctor
- Children under 12 years: Do not use without consulting a doctor
Other Information
- This product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- This product may stain contact lenses and other lis if handled after touching tablets.
- Store at room temperature between 15°C -30°C (59°F -86°F) in a dry place and protect from light.
Inactive Ingredients
colloidal silicone dioxide, gum acacia extra pure, hydroxy propyl methyl cellulose, lactose monohydrate, magnesium stearate, maize starch, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, sodium starch glycolate
Questions Or Comments?
call 1-800-632-6900
DISTRIBUTED BY THE KROGER CO.CINCINNATI, OHIO 45202
Principal Display Panel - 95 Mg Tablet Blister Pack Carton
*Compare to active ingredient of AZO® URINARYPAIN RELIEF MAXIMUM STRENGTH *see back panelNDC XXXXX-XXX-XX
UrinaryPain Relief
PhenazopyridineHydrochloride 95 mg
FAST RELIEFFOR URINARYDISCONFORT
actualsize
30 TABLETS
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


