Vancomycin 250 MG Oral Capsule
1 INDICATIONS AND USAGE Vancomycin hydrochloride capsules are indicated for the treatment of Clostridioides difficile -associated diarrhea. Vancomycin hydrochloride capsules are also used for the treatment of enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) in adult and pediatric patients lessthan 18 years of age. Limitations of Use • Parenteral administration of vancomycin is not effective for the above infections; therefore, vancomycin hydrochloride capsules must be given orally for these infections. • Orally administered vancomycin hydrochloride capsules are not effective for other types ofinfections. To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin hydrochloride capsules and other antibacterial drugs, vancomycin hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Vancomycin hydrochloride capsules are glycopeptide antibacterial indicated in adult and pediatric patients (less than 18 years of age) for the treatment of: ( 1 ) Clostridioides difficile -associated diarrhea Enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) Limitations of Use: ( 1 ) ( 5.1 ) • Parenteral administration of vancomycin is not effective for the above infections; therefore, vancomycin hydrochloride capsules must be given orally for these infections. • Orally administered vancomycin hydrochloride capsules are not effective forother types of infections. To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin hydrochloride capsules and other antibacterial drugs, vancomycin hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. ( 1 )
alvogen inc.
Related Pills
Vancomycin 125 MG Oral Capsule
alvogen inc.
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site





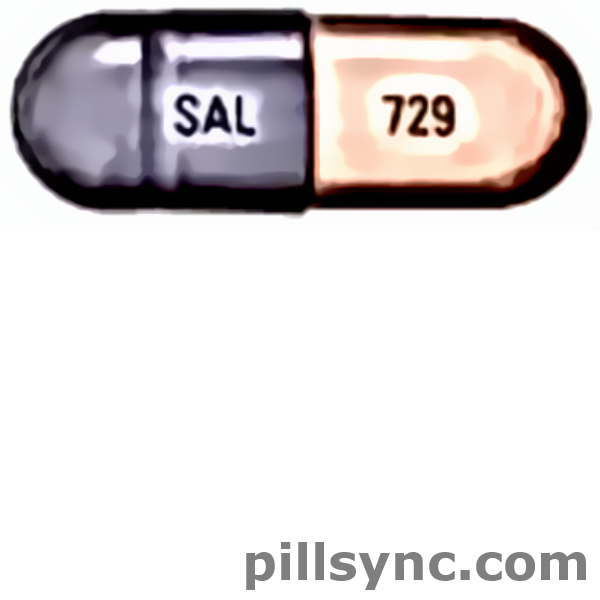
16 HOW SUPPLIED/STORAGE AND HANDLING
VANCOMYCIN HYDROCHLORIDE capsules, USP are available in: The 125 mg* capsules have a grey cap and pink body imprinted with "SAL" on the cap and "729" on the body in black ink. The 250 mg* capsules have a brown cap and brown body imprinted with "SAL" on the cap and "730" on the body in white ink. Strength Pack NDC number Vancomycin 125 mg Blister pack of 20 47781-729-02 Bottle pack of 50 47781-729-50 Bottle pack of 100 47781-729-01 Vancomycin 250 mg Blister pack of 20 47781-730-02 Bottle pack of 50 47781-730-50 Bottle pack of 100 47781-730-01 *Equivalent to vancomycin. Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F) [see USPControlled Room Temperature].
More pills like CAPSULE SAL 730