clindamycin 150 MG Oral Capsule
INDICATIONS AND USAGE Clindamycin hydrochloride capsules are indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Clindamycin hydrochloride capsules are also indicated in the treatment of seriÂous infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, as described in the BOXED WARNING , before selecting clindamycin, the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin). Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastroinÂtestinal tract); infections of the female pelvis and geniÂtal tract such as endometritis, nongonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection. Streptococci: Serious respiratory tract infections; seriÂous skin and soft tissue infections. Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections. Pneumococci: Serious respiratory tract infections. Bacteriologic studies should be performed to deterÂmine the causative organisms and their susceptibility to clindamycin. To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteÂria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Aurobindo Pharma Limited
Related Pills
clindamycin 300 mg
Aurobindo Pharma Limited
Clindamycin 150 MG Oral Capsule
Aurobindo Pharma Limited
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site







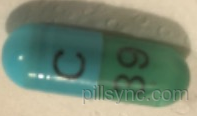
HOW SUPPLIED
CLINDAMYCIN HYDROCHLORIDE capsules USP, 150 mg are light blue opaque/light green transparent size ‘1’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘C’ on light blue opaque cap and ‘39’ on light green transparent body with black ink. Bottles of 100 NDC 65862-185-01 Bottles of 500 NDC 65862-185-05
CLINDAMYCIN HYDROCHLORIDE capsules USP, 300 mg are light blue opaque/light blue opaque size ‘0’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘C’ on light blue opaque cap and ‘40’ on light blue opaque body with black ink. Bottles of 16 NDC 65862- 186 -16 Bottles of 100 NDC 65862-186-01 Bottles of 500 NDC 65862-186-05 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Pharmacist: Dispense in a tight container with child-resistant closure.
More pills like CAPSULE C 39